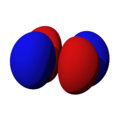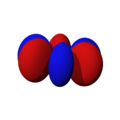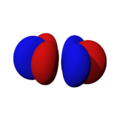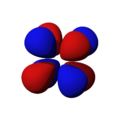Appendix C6 - MO Theory - Orbital Shapes
Although the subject of electronic orbitals can be quite complicated and is pushing you into the realms of high-end A-level and first-year undergraduate level, a brief explanation of the currently accepted model will be given here for reference.
Atoms contain electrons, a neutral atom will contain as many electrons as there are protons in the nucleus. Electrons, being a negatively charged and protons being positively charged will naturally attract towards each other and it was Neils Bohr (mentioned previously) who put forward the theory of energy levels which would prevent the electrons from simply smashing into annihilation against the nucleus of the atom. But what do these energy levels actually look like, how can we explain or represent them?
The orbitals represent different energy levels, the closer the orbital is to the nucleus the lower its energy level. Electrons aim for the lowest possible energy level and so as the nucleus grows in size and therefore the number of electrons increases, the electronic orbitals fill from lower to higher energy. The outermost energy levels are occupied by the electrons which take part in chemical bonding, these are usually referred to as the "valence" or "valency" electrons.
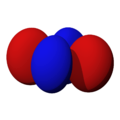
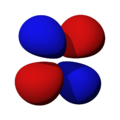
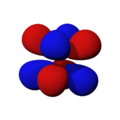
The shapes of the orbitals are intended to represent a mathematical probability of where an electron is likely to be found at any given time. The orbitals are noted as "s", "p", "d" and "f" respectively and are given shapes as shown in the diagrams.
|
1 |
s |
|
|
|
|
|
|
|
|
s |
|
|
|
|
|
2 |
p |
|
|
|
|
|
|
|
|
px |
py |
pz |
|
|
|
3 |
d |
|
|
|
|
|
|
|
|
dx2-y2 |
dxy |
dxz |
dyz |
dz2 |
|
4 |
f |
|
|
|
|
|
|
|
|
fx(x2-3y2) |
fxyz |
fxz2 |
fy3x2-y2 |
fyz2 |
|
4 |
f |
|
|
|
|
|
|
|
|
fz(x2-y2) |
fz3 |
|
|
|
Back To >> What Causes Magnetism ? <<