Carboxylic Acids
The carboxylic acids are a group of compounds which contain one or more of the carboxylic groups - COOH:
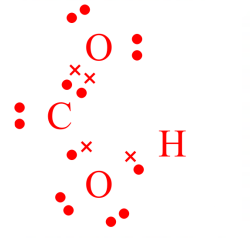
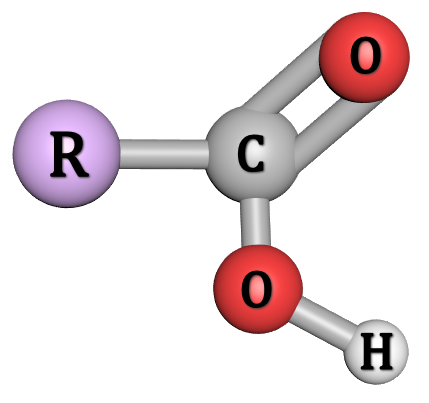
The "dot and cross" diagram and the three-dimensional "ball and stick" models show the orientation of the carbon, hydrogen and oxygen atoms making up this functional group. The "carboxylate" group carries an overall single negative charge (in, for example Sodium Ethanoate).
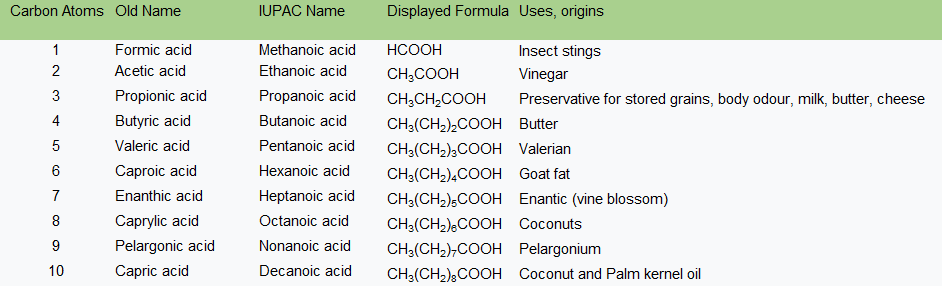
The central carbon atom, the one which is double bonded to the one oxygen atom and single bonded to the hydroxyl group, is the atom which carries the bond to the remainder of the molecule making up the acid. The simplest carboxylic acid has a single hydrogen atom as the "remainder of the molecule", and this is known as Methanoic Acid. In just the same way that a hydrogen atom was dropped off, say a methane molecule to make a "methyl" group, we name the simplest carboxylic acids using the alcohol group prefix by removing the "yl" and replacing this with "anoic" to make the name of the acid.
Some of these acids are still known by their "old name" so it is useful, but not necessarily compulsory, that you are familiar with these old names as you may come across them from time to time in some older textbooks.
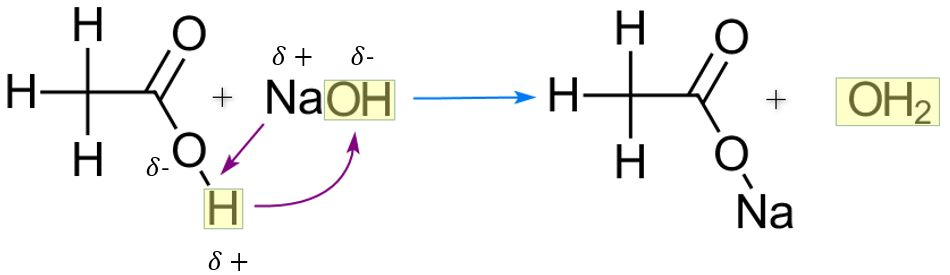
We can have metal salts of carboxylic acids, if we react for example ethanoic acid with sodium hydroxide, or sodium hydrogen carbonate (aka sodium bicarbonate) (a typical acid plus base reaction) we produce an aqueous solution of sodium ethanoate and water (a typical salt plus water response to the previous).
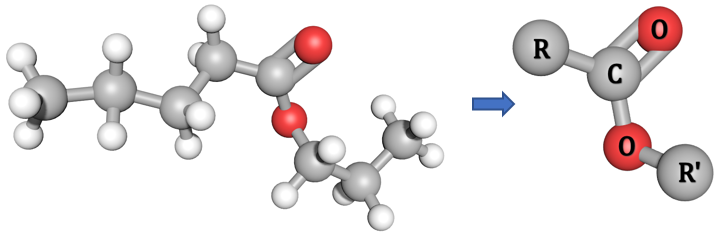
You will find in your studies of organic chemistry that alkyl groups are sometimes abbreviated to R, or R' and so forth. For the smaller molecules this isn't such a problem, but if you are asked to describe or represent the displayed (or 3D) formula of a particularly big molecule (such as, say, the ester Propyl Pentanoate (below)) you will find it easier to use the R and R' representations:
Go To >> Appendix C9 - Organic Nomenclature Substituent Group Hierarchy <<