Isotopes
We know that atoms of the same element contain the same number of protons, but the same cannot necessarily be said about the number of neutrons.
For example the element carbon has three different "types" of itself, carbon-twelve, carbon-thirteen and carbon-fourteen. We have established that the atomic number of carbon is six, therefore there are six protons in the nucleus and this can never change (otherwise carbon would cease to be carbon!). But the differences in the relative atomic masses give us examples of carbon with six, seven and eight neutrons respectively. These differing versions are called isotopes of the element.
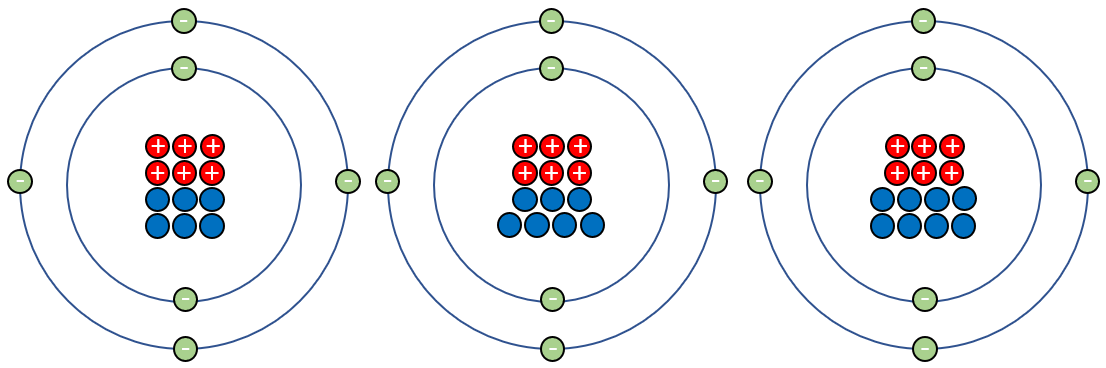
In the diagram above we can see that in carbon twelve we have six neutrons, but in the remaining isotopes we have seven and eight respectively. The element does not cease to be carbon because the number of protons remains the same at six, similarly the number of electrons remains at six as the isotopes are all electrically neutral (don't forget, neutrons carry no charge so make no contribution to the overall electrical status of the atom).
Calculations involving isotopes can become quite tricky, let's have a look at an example:
Q. if we consider the three isotopes of carbon, we can quickly establish that carbon twelve has a relative abundance of 98.89%, whereas carbon thirteen has a relative abundance of 1.109% and finally carbon-14 has a relative abundance of one part in 1 trillion or represented as a percentage approximately 0.000000000001 % which is so small as to be almost insignificant, but for the purposes of this calculation will be included anyway.
A. The first thing to do, (and indeed I consider this to be the best way) is to convert the percentages to decimal numbers, so for example 98.89% becomes 0.9889, 1.109% becomes 0.01109 and the final figure for carbon-14 becomes 0.00000000000001. We achieve this by dividing the percentages by 100. Within multiply each percentage by the atomic mass of the particular isotope, so in the case of carbon twelve we have 12 x 0.9889 and for carbon thirteen we have 13 x 0.01109. Finally for carbon-14 we have 14 x 0.00000000000001.
If we now perform these calculations and add up the totals we will end up with a relative atomic mass (average mass) for the element carbon:
![]()
If you look at the periodic table you will see the relative atomic mass of carbon stated as 12.011 so you can see that this calculation is quite accurate.
Q. Chlorine exists in mainly two isotopes, chlorine thirty-five and chlorine thirty-seven. The percentage abundance of chlorine thirty-five is 75.77% whilst the percentage abundance of chlorine thirty-seven is 24.23% (there is an isotope chlorine thirty-six but its abundance is infinitesimal and usually disregarded for the purposes of these types of calculations). With this information calculate the relative atomic mass of chlorine.
A. As before, the first step to take is to convert the percentages that you've been given into decimals by dividing each one by hundred. 75.77 therefore become 0.7577 and 24.23 therefore becomes 0.2423. We now multiply these numbers by the respective atomic mass of the isotope that they refer to us:
![]()
On any periodic table you care to look at, the relative atomic mass of chlorine is stated as 35.5 so you can see once again that these calculations are quite accurate.
Q. Copper has two stable isotopes, copper sixty-three with an abundance of 69.2% and copper sixty-five with an abundance of 30.8%. Calculate the relative atomic mass of copper.
A. I will go straight to the solution, if you can't understand this please work through the previous examples:
![]()
Q. Boron ten has an abundance of approximately 19.9%, its stable isotope "sister" boron eleven makes up the rest of the abundance of this element. With this information, calculate the relative atomic mass of the element boron.
A. The only thing that hasn't been given to here is the abundance of the second isotope, this is easy to calculate because the total abundances have to equal 100% so the abundance of the second isotope is simply 100-19.9 or 80.1%. Now that we have suitable information I will once again go straight to the answer:
![]()
Q. The group 2 metal magnesium exists in three stable isotopes, magnesium twenty-four, magnesium twenty-five and magnesium twenty-six. These isotopes have relative abundances of 79%, 10% and 11% respectively. Calculate the relative atomic mass of magnesium.
A. Once again, straight to the calculation and result:
![]()
Questions that you might see could include asking you to give a short explanation as to why the relative atomic mass of a particular element is not a whole number, for example copper at 63.5 and chlorine, as we have already seen, at 35.5. This is due to the relative abundances of the different isotopes and calculations such as the ones above will produce the arithmetical reason why.
Similarly, a sneaky examiner might ask you to calculate the atomic mass of a particular isotope given all of the rest of the information you will need. To calculate this I'm going to "reverse engineer" the calculation we did previously for copper. If we consider the unknown atomic mass as a variable "X" as we might in algebra, we would be able to construct an algebraic expression which can then be transposed in terms of X to obtain the relevant value. First of all let's see what the question might look like:
Q. Copper exists in two stable isotopes, copper sixty-five with an abundance of 30.8% and another isotope. Given that the relative atomic mass of copper is 63.6, calculate the atomic mass of the unknown isotope.
A. First of all, study what you've actually been given and then decide what you can quickly work out. You are told that the abundance of one of the isotopes and that there are only 2 to consider anyway, with this in mind you can quickly work out that the abundance as a percentage of the second isotope would have to be 100-30.8 or 69.2%. Let us now reconstruct our calculation as above inserting an algebraic variable in the place of the unknown isotope mass:
![]()
Stop at this point and study the expression, in previous examples you have taken the percentages and turn them into decimals. You have done nothing different here, the percentages of 30.8 and 69.2 have been turned into the decimal equivalents of 0.308 and 0.692. The only value that we haven't got is the atomic mass of the second isotope which we now refer to as X. What we need to do now with this expression is to rearrange it in terms of X, so we can then evaluate X. First of all let us look at what we can do:
We can multiply 65 x 0.308 to give us a value of 20.02, so now we can rearrange our equation thus:
![]()
Let us now perform a simple algebraic manipulation, we can either call it "moved 20.02 across to the right-hand side" or probably more sensibly "subtract 20.02 from both sides" this will give us the following expression:
![]()
Now we simply divide both sides by 0.692:
![]()
You will probably be asked to leave your calculation either as an integer value or to one decimal place, in this particular question as your first isotope was given as an integral value I would expect that you would be asked to round your answer to the nearest whole number, which gives sixty-three as above.
>> Questions <<