Osmosis
Following on from "diffusion", we look at another type of intracellular transport known as "osmosis". Osmosis is an important process for life and is defined as:
"Osmosis is the movement of water molecules across a partially permeable membrane from a region of
higher water concentration to a region of lower water concentration"
You could regard osmosis as a specific type of diffusion, involving only water. In other textbooks you may see "partially permeable membrane" referred to as a "semipermeable membrane", usually in much older textbooks but you may still encounter it.
Think of your partially permeable membrane as a tea sieve but one which has holes in it so small that only water molecules can pass through, larger molecules such as sugars are too big to pass through the holes.
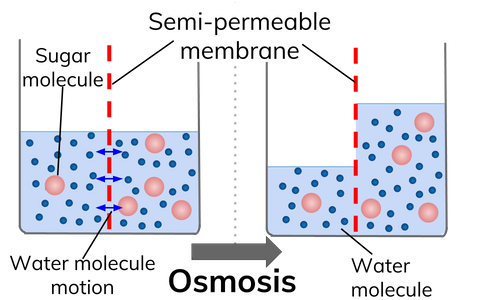
Osmosis can be thought of as a method by which the concentrations of water either side of a "divide" as it were is trying to be "evened up". The famous potato stick experiment is one that you will conduct at school to show the effects of varying concentrations of sugar solutions on a cylinder of raw potato.
Animal and plant cells lose or gain water by osmosis to maintain their natural balance.
(Left) If a cell starts to become depleted of water, the concentration of water inside the cell will start to drop below the concentration of water outside the cell and as a result of this water will diffuse across the partially permeable membrane by osmosis into the cell to address the balance.
(Right) Similarly if a cell starts to contain too much water, its concentration will start to exceed that of the surroundings at which point osmosis will cause water to diffuse out of the cell.......
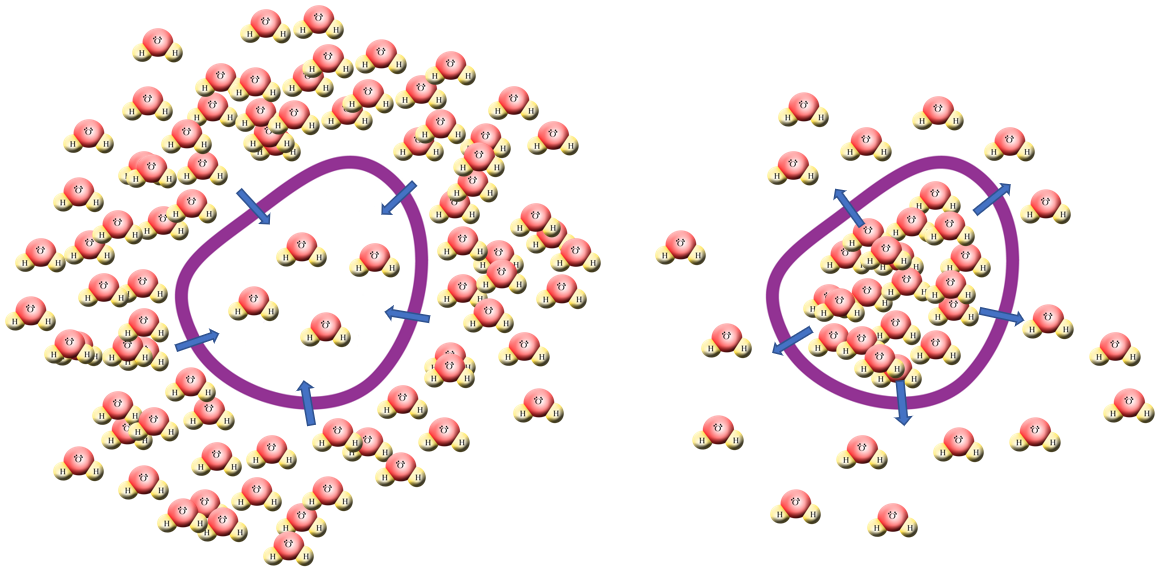
>> Questions <<