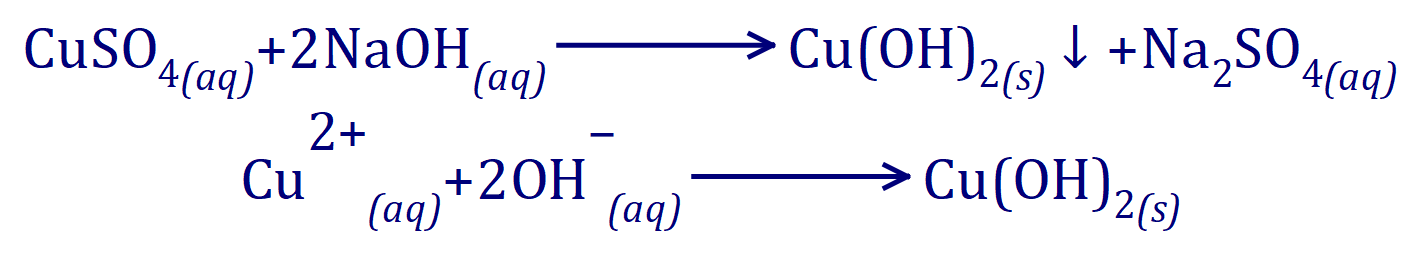Tests for Positive Ions (Cations) - Hydroxide Precipitates
The hydroxides of a number of metals are insoluble in solution and will form precipitates. Some of these hydroxides have quite characteristic colours and often it is possible to identify the cation present by the colour of the hydroxide which precipitates out.
For example if we add a small amount of Sodium Hydroxide solution to a solution of a copper salt (eg: Copper (II) Sulphate) we will produce a blue precipitate of Copper Hydroxide:
|
|
|