[A] Enthalpy and Entropy
This particular section could get quite long, it is a particularly advanced section as the topics that will be discussed are usually found on the GCSE A-level syllabus here in the United Kingdom. We are going to talk about Enthalpy, Entropy and free energy. The first two subjects will talk about changes during a chemical reaction and the third talks about determining the feasibility of the reaction, i.e. whether it will take place or not.
Before we start talking about these, it will be as well to give definitions of what they are:
Enthalpy - this is a measurement of the energy in a thermodynamic system, it is the thermodynamic quantity equivalent to the total heat content of the system. There are many different types of enthalpy, such as enthalpy of combustion, enthalpy of formation and these are all to do with energetic changes in the system during, or as a result of, a chemical reaction. Enthalpy is measured in kilojoules per mole.
Entropy - this is usually defined as the “degree of disorder” in the system, that is the more disordered the system is the greater the entropy. Entropy is measured in Joules per Kelvin per mole.
Free Energy - the energy that can be converted into work at a constant temperature and pressure throughout a system. Free Energy is measured in kilojoules per mole.
We will first talk about enthalpy, which is basically a measurement of energy. When chemical reactions occur there will be a change in energy, and in thermochemistry and energetic terms this is known as an “enthalpy change”.
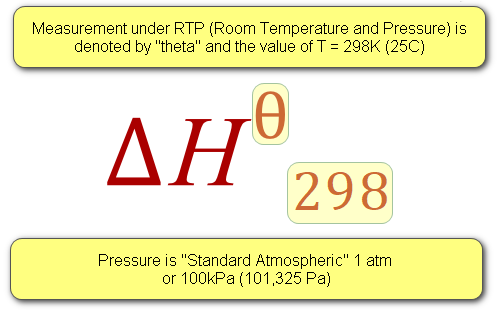
Enthalpy change is usually denoted as a triangle with a H next to it, known as “Delta H” and is the heat energy transferred in the reaction measured at a constant pressure. Let’s take a look at a couple of symbols now so that we can get our heads around this before I carry on to the more complicated bits.
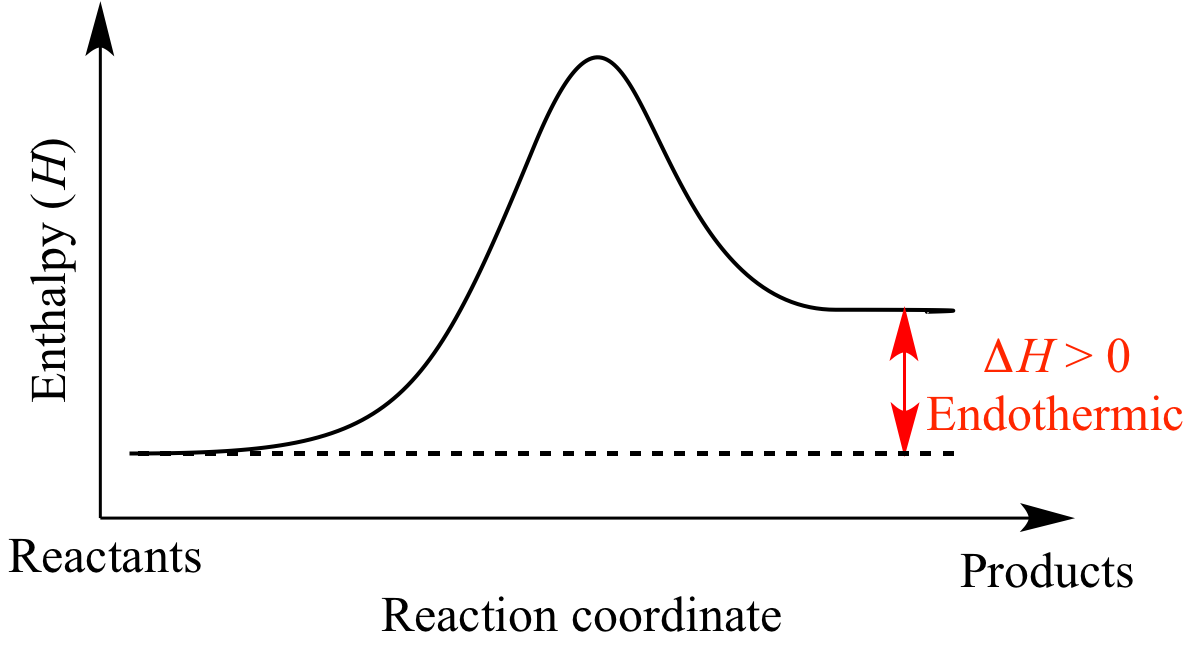
Enthalpy changes are measured in kilojoules per mole, and energy changes can be positive or negative. If, during a chemical reaction energy is taken in (meaning that the products of the reaction contain more energy than the reactants) then the enthalpy change will be positive and this is said to be an endothermic reaction.
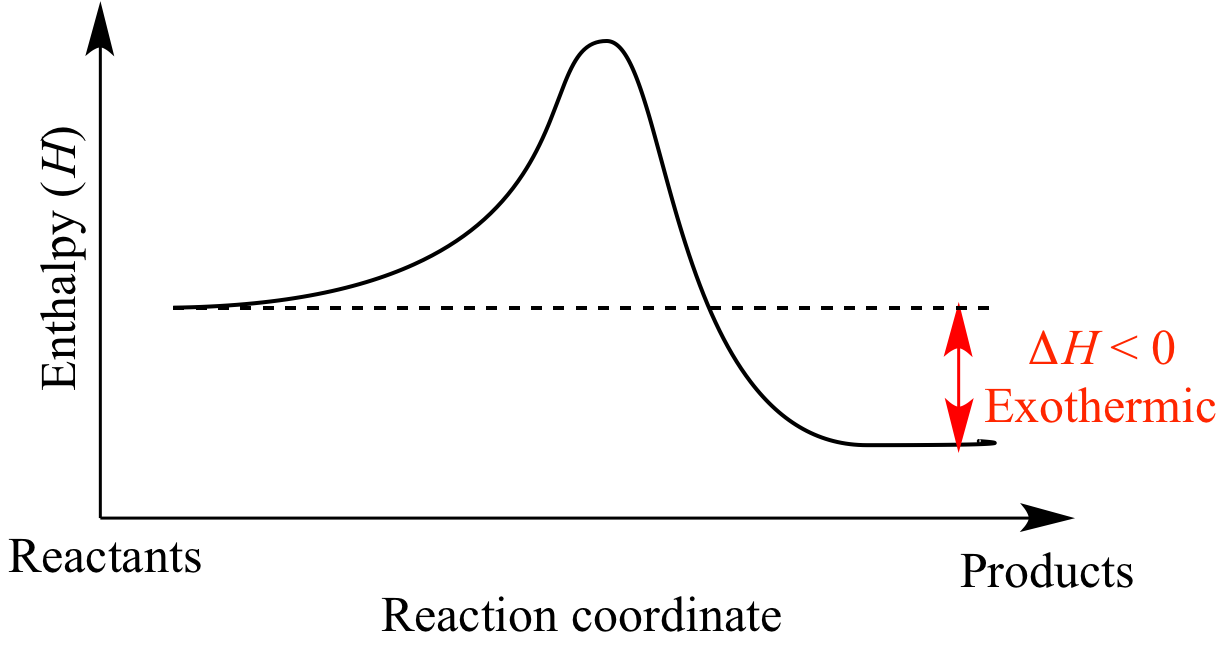
In any reaction where the enthalpy change is negative, that is the products contain less energy than the reactants, then for this to be the case energy must have been given out to the surroundings, in such cases this is said to be an exothermic reaction.
If the energy involved in breaking bonds is more than that involved in making them, then the reaction will need to take in energy from the environment and will therefore be endothermic .
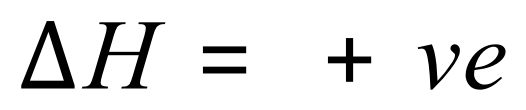
If the reverse applies, and the energy involved in breaking the bonds is less than that given out when new bonds are made, there will be an excess of energy which will be given out to the environment in an exothermic reaction.
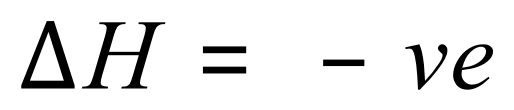
We talk about bond enthalpy, in days gone by (in older chemistry textbooks) you would have seen this referred to as bond energy, and despite the fact that the name is changed, the meaning is still the same. Atoms in molecules are held together by very strong covalent bonds and it takes energy to break these bonds. Bond enthalpy is always positive as energy is needed to be applied to break the bond, i.e. bond breaking is an endothermic process.
Chemistry textbooks usually have tables of bond enthalpy values, but these will vary depending on the environment of the bond, or in other words the energy required to break a particular bond will depend on the environment that that particular bond finds itself in.
For example, let us consider the water molecule which contains two O-H bonds, you would be forgiven for thinking that the energy to break them both would be identical because the molecule is symmetrical, but once one of the bond is broken it is slightly easier to break the second one because when the first hydrogen leaves the molecule, it does so as a proton, leaving behind its electron and what remains is a hydroxide ion.
This hydroxide ion contains an overall negative charge, and electron repulsion makes the remaining OH bond a little bit less stable than the first one was:
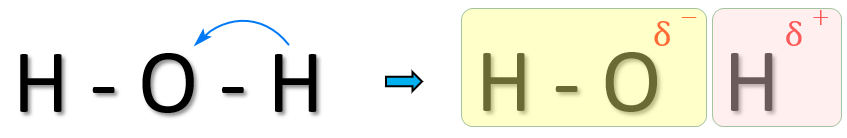
Picture the water molecule on the left-hand side. During the breaking of the right-hand OH bond, the hydrogen atom’s electron is transferred to the remaining (soon to be) hydroxide ion. Electron repulsion within the remaining OH bond means that this one will break easier than the first.
This accounts for the fact that the first bond requires 492 kJ per mole whereas the second bond requires only 428 kJ per mole. You will therefore often find an average, or ‘mean’ value quoted, which in this particular case is 460 kJ per mole but this may vary between texts, as there are many other types of OH bond and values quoted for the ‘mean’ will include these other examples (such as OH bond in alcohol and carboxylic acid groups etc).