The Energetics of Photosynthesis
To close this section (for a while anyway) I would like to have a look at a considerably difficult example, the photosynthesis of Glucose from Carbon Dioxide and Water. Just as in the previous examples this involves breaking and making of bonds, each of which takes in or releases energy, however there are a LOT of bonds to consider and a displayed formula here would be essential:
Take a look at the symbol equation:
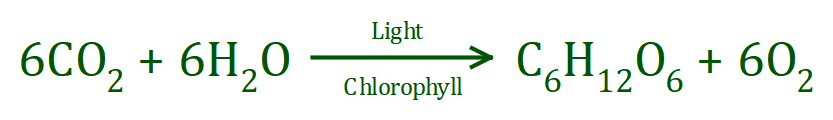
The size of the glucose molecule produced should tell you that a structure is needed, just to be able to visualise the bond breakage and formation. Lets look at the reactants:
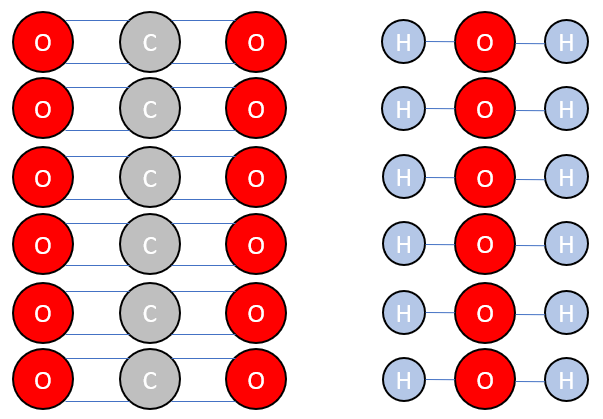
From the symbolic equation it's not quite clear enough how many bonds are involved, but when you break it down diagrammatically you can see that the formation of one molecule of glucose requires the breakage of twelve "carbon to oxygen" double bonds and the twelve "hydrogen to oxygen" single bonds.
C=O = 799 kJmol-1
H-O = 467 kJmol-1
The products are somewhat different:
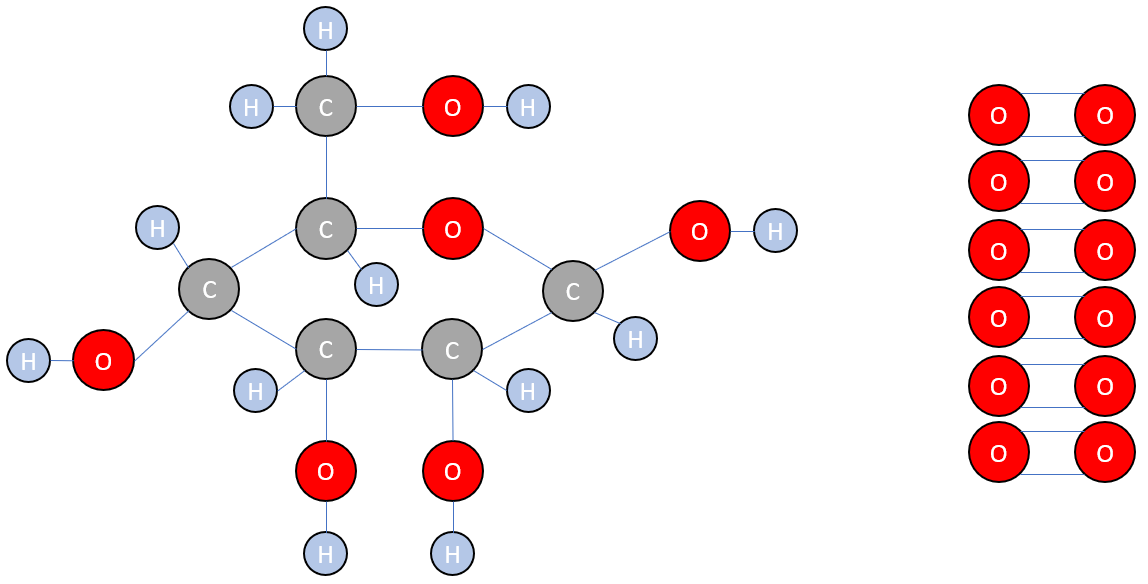
A little bit of very careful study will tell you that to produce one molecule of glucose requires the formation of 5 "carbon to carbon" single bonds, 7 "carbon to oxygen" single bonds, 5 "hydrogen to oxygen" single bonds and 7 "carbon to hydrogen" single bonds. In addition, we should not forget the fact that we also produce 6 "oxygen to oxygen" double bonds.
** It should be REMEMBERED that when we actually carry out the calculations, we are talking about molar quantities and not individual molecules. **
C-O = 358 kJmol-1
H-O = 467 kJmol-1
C-C = 347 kJmol-1
C-H = 413 kJmol-1
O=O = 498 kJmol-1
Now that we know how many bonds are broken, and how many are formed, we can proceed to the calculation.
Bonds broken (in kJmol-1) :
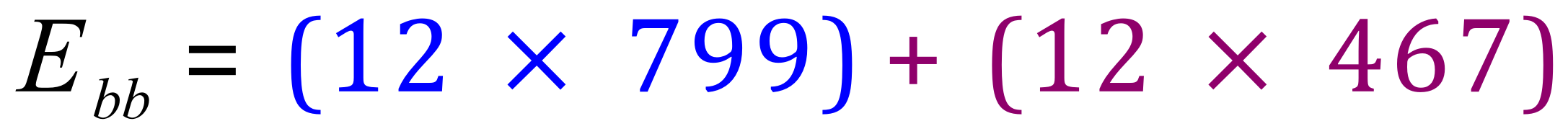
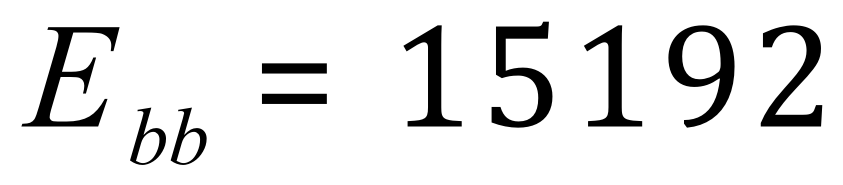
Bonds formed (in kJmol-1) :

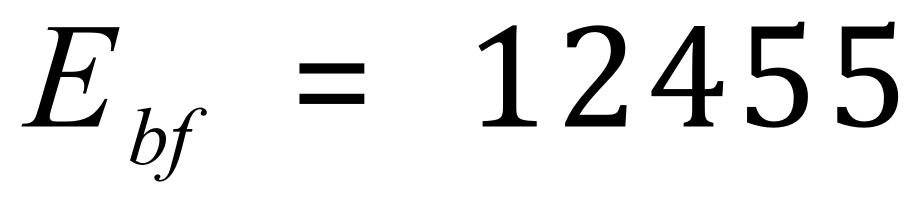
The overall energy (enthalpy) change is therefore:
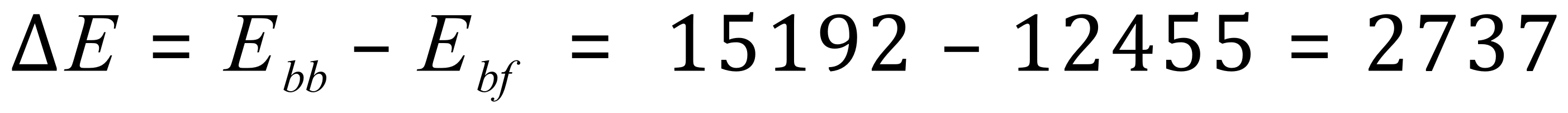
This is a positive value because more energy was required to break the bonds than was required to form the bonds in the products. Therefore this is a quite highly endothermic reaction.
NB: I have used Ebm and Ebf in these pages, these are synonymous (bonds made = bonds formed).