Energy Changes In Reactions
Now we can move on to the creation of compounds, in terms of the energy requirements for bond making and breaking.
Example 1:
Let's take a look at a simple calculation involving the use of bond energies, the simple reaction between hydrogen and chlorine to produce the gas hydrogen chloride.

If we just "say" the equation, it would go like this "one mole of hydrogen molecules reacts with the one mole of chlorine molecules to produce two moles of hydrogen chloride molecules". If we are given the bond energies of hydrogen and chlorine, and the bond energy between a hydrogen and chlorine atom in the newly created hydrogen chloride molecule we can work out the energetics of the reaction and see whether or not it takes in or gives out energy.
Unless you are very confident with these types of calculation my strongest advice is to draw out the displayed formula of each reactant and product, in the correct molar amounts so that you can see at a glance what bonds are being broken, what bonds are being formed and in what number.

We can see from the displayed formula representation that one mole of H-H bonds and one mole of Cl-Cl bonds are broken, and two moles of H-Cl bonds are formed. Values that we need are: Bond Energy : H-H = 436 kJ/mol, Cl-Cl = 242 kJ/mol and H-Cl = 431 kJ/mol. From all the information that we have we can work out that the input energy is 436+242= 678 kJ per mole and that the energy released during the making of the product bonds is 2×431= 862 kJ per mole.
The overall energy change during this reaction is therefore:

This can be explained by saying that "Delta E" (the energy change in the reaction) is the product of the energy used in breaking bonds - the energy released in the making of new bonds. In this particular case more energy is released in the making of the new bonds than in the breaking of the old bonds, so the calculation is negative which means that the reaction releases energy, i.e. it is exergonic (we usually say exothermic because the excess energy is more often released as heat, but this doesn't always have to be the case so the correct expression is exergonic).
The equation to remember in these cases, and if you do it will always give you the correct answer is this:
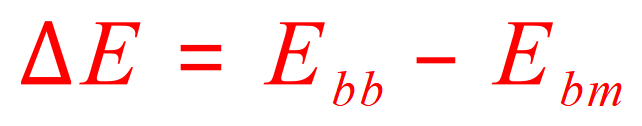
"The change in energy is equal to the energy required to break the bonds - the energy released when bonds are made"
These calculations are relatively straightforward provided that you are structured and methodical in the way that you approach them and all of the stages. Until you are superbly confident in your abilities you should always write out the intermediate steps, including the displayed formulae and numbers of molecules involved this way you shouldn't go wrong when calculating the numbers of bonds involved.
Example 2:
Methane burns in oxygen according to the following equation:
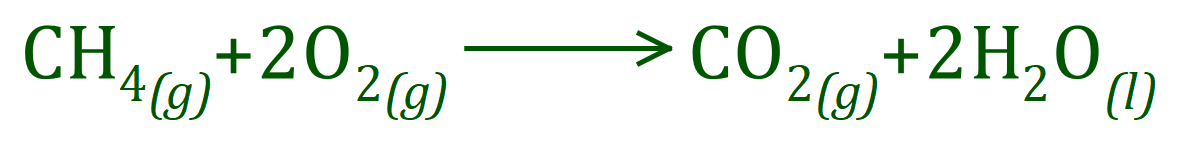
The overall energy change (the enthalpy change) for the reaction is highly exothermic (exergonic, but in this case we can say exothermic because we know it is heat energy given out). Given the bond energies below, calculate the enthalpy change for the reaction in kilojoules per mole.
C-H 413kJ/mol, O=O 498kJ/mol, C=O 805kJ/mol and O-H 464kJ/mol.
First of all, draw the displayed formula for each molecule involved:
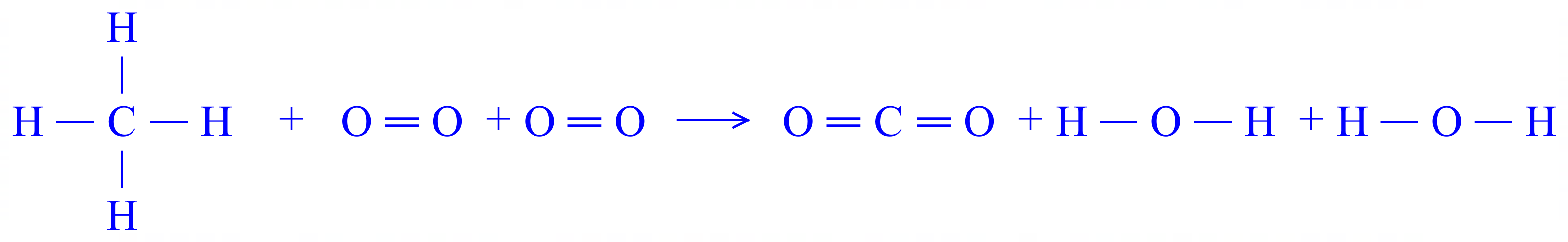
This makes it easier to see that you will need to break 4 C-H bonds, 2 O=O bonds (don't forget this is molar quantities). and you will form 2 C=O bonds and 4 O-H bonds.
Now that you know which bonds will be broken, which bonds will be formed and in what numbers you can start looking at the numbers:
Bonds broken = 4 x C-H (4 x 413) = 1652
Bonds broken = 2 x O=O (2 x 498) = 996
The total energy required to break all of the bonds necessary is therefore 1652+996 = 2648 kJ/mol
Bonds made = 2 x C=O (2 x 805) = 1610
Bonds made = 4 x O-H (4 x 464) = 1856
The total energy released when the new bonds are made is therefore 1610+1856 = 3466 kJ/mol

As you can see, a highly exothermic reaction!
You may find that in some questions, you will be given the overall enthalpy change and all but one of the bond energies. Your task in this sort of question will be to calculate a value for the missing bond energy. Let's take a look at an example:
Example 3:
Methanol burns in oxygen (air) to produce carbon dioxide and water, according to the equation below:

The overall enthalpy change (Delta H) for the reaction is -1316 kJ/mol, highly exothermic. Given the bond energies below, calculate the value for the bond energy between the C and O in Carbon Dioxide.
C-H = 413 kJ/mol
C-O = 358 kJ/mol
O-H = 464 kJ/mol
O=O = 498 kJ/mol
C=O = "x"
This is slightly more complicated than previous ones, but we approach it in exactly the same way by first of all showing the "displayed formula" for each reactant and product concerned, and in the appropriate quantities (how you choose to do this is entirely up to you, but make sure that you do it otherwise some or all of your calculations will be thrown out).
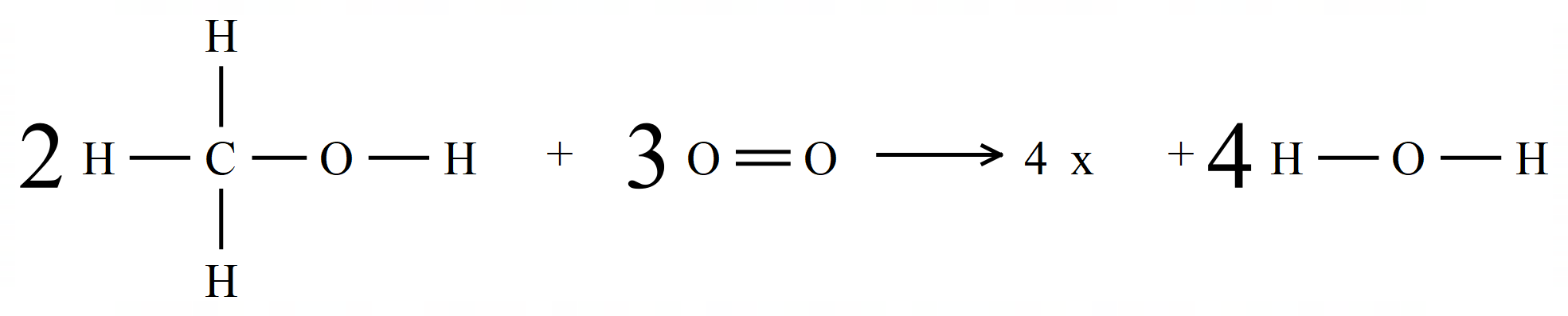
Stop and study the displayed formula for each compound and you should quickly come to the conclusion that you will break 6 CH bonds, 2 CO bonds, 2 OH bonds and 3 OO bonds. Let's deal with these first.
6 CH bonds = 6 x 413 = 2478 kJ
2 CO bonds = 2 x 358 = 716 kJ
2 OH bonds = 2 x 464 = 928 kJ
3 OO bonds = 3 x 498 = 1494 kJ
The sum of all of the broken bonds comes to 5616 kJ. This is the total energy required to break the relevant bonds. Now we take a look at part two, the formation of the new bonds.
4x = This is 4 of the new bonds that we have been asked to calculate the value for, so we've given it an algebraic variable of 'x' just for simplicity.
8 OH bonds = 8 x 464 = 3712 kJ
Stop and study this for a while. The energy produced in the formation of the new products is 3712 kJ for the water (pay special attention to the fact that although we only have 4 molecules of water we have 8 of the bonds required). We produced 2 molecules of carbon dioxide, so 2×2 = 4 of the new bonds for which we need to calculate the bond energy 'x'.
The sum of all of the newly created bonds comes to 4x+3712 kJ. As we already know the overall enthalpy for the reaction, we can substitute the values that we have to produce the following equation. What we need to do now is rearrange this equation in terms of 'x' to deduce the value for the bond concerned:
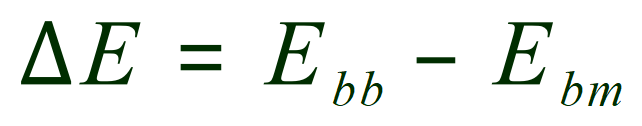
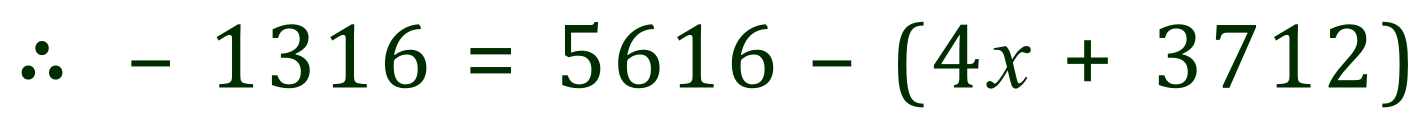
Rearranging (in stages, follow these carefully):

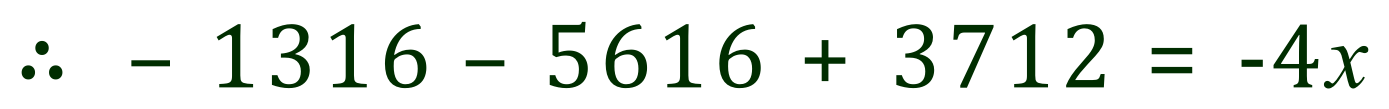
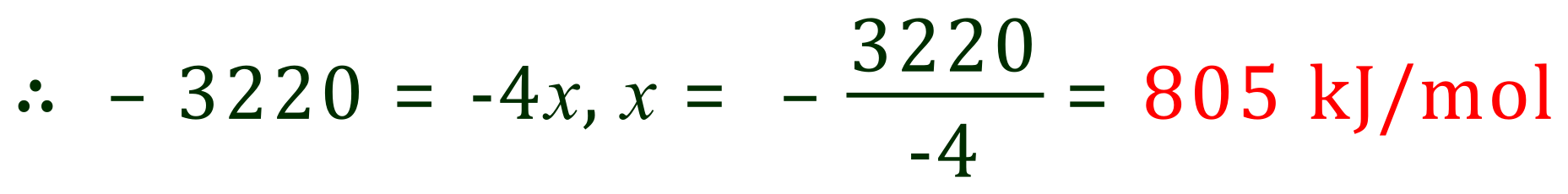
The bond enthalpy (energy) for the C=O bond in Carbon Dioxide is therefore 805 kJ/mol. If you look at the table of bond energies in Appendix C5 you will see a different value, there is always some variation in these calculated values and it is unlikely that you will see many agreements on data sources. Always use the values provided in your question to avoid any problems.
>> Questions <<