Bond Breaking and Bond Formation
It's been mentioned previously in this book that the protons in an atom define its identity but it's the electrons that provide the character. Certainly it is the electrons in an atom which effectively do all of the work and are responsible for the making or breaking of chemical bonds to produce new compounds. Making and breaking chemical bonds requires either the input or output of energy and this section will talk about some of the energy changes involved.
As we know, energy is measured in Joules but some chemical reactions can involve the transfer of thousands or even millions of Joules so we need to get used to seeing the expression "Kilojoule" and "Megajoule" and we will see this in connection with amounts of reactants and products involved. I'm sure you can understand that for example an explosion involving 1 g of gunpowder would be slightly different to one involving a ton of gunpowder, in the latter case far more energy would be involved and so it is usual to see the amount of energy expressed as a ratio to fixed amounts which, in chemistry we usually refer to as the "mole". If you need a refresher on what a mole is then you should flick back to the section >> The Mole << before you carry on reading.
In chemical reactions there is an "energy transfer" and I've tried to be careful to this point not to say which way it goes, because energy can be expelled from the system or absorbed into the system and there are special names for these types of energy transfer.
Endothermic - An endothermic reaction is a reaction which requires the input of energy,
the breaking of chemical bonds requires energy and is therefore endothermic.
Exothermic - An exothermic reaction is a reaction in which energy is released into the system.
When chemical bonds are formed, energy is released into the system and so bond formation is an exothermic process.
There are many factors to consider when chemical bonds are made or broken, how easy or difficult it is to break the bond depends on how enthusiastic (or otherwise) one or both of the atoms involved in the bond is to release, accept or share an electron.This gives rise to the reason that some bonds are easy to break than others, and the amount of energy required to break a bond (which is coincidently the same amount of energy required to make the same bond) is known as the "bond energy" and is measured in kilojoules per mole. We don't normally talk about the energy required to produce or break a single bond, we look at the energy required to produce or break a mole of the bonds.
Even the "same bond" (eg: C-O) can have a slight variation in its reported bond energy because this is dependent on the compound that it is forming part of, and you will see variations in reported values depending on the book you're looking at or website you're looking at. If you are required to perform any bond energy calculations, you will be given the necessary bond energies and you should use the ones provided.
There are a number of ways to show the "structure" of a molecule, the simplest is the displayed formula which shows the atoms by their atomic symbol and a single double or triple line depending on the type of bond between them:

This is the displayed formula for a simple hydrogen molecule, if you wish to see an alternative representation which gives a little bit more information of the bond between the two you can use The "dot and cross" representation which emphasises the fact that electrons are shared in the bond (in the case of a covalent bond) and one electron is provided by each atom.

436 kJ/mol energy required to break 1 mole of H-H bonds
Of course this is just a model, we can't say that the "cross" electron came from the right-hand hydrogen and the "dot" electron came from the left-hand hydrogen, it's not possible to do this. This representation is purely for simplicity but is quite powerful in its use. The "bond energy" for a hydrogen molecule is in fact 436 kJ per mole which means that 436,000 J of energy would be required to break one mole (the Avogadro number) of hydrogen to hydrogen bonds, ergo one mole of hydrogen molecules.
Some atoms are more enthusiastic than others when it comes to accepting or donating electrons and so their individual "atom to atom" bond energies may be different.
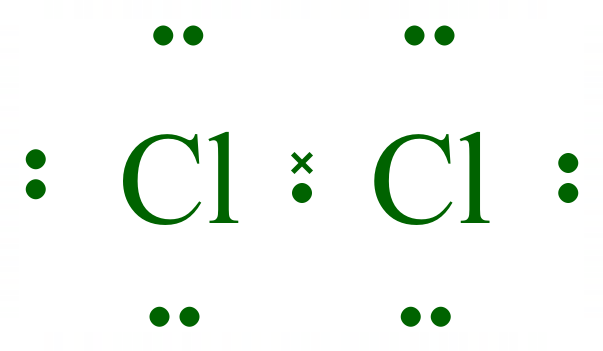
In a diatomic chlorine molecule, each chlorine atom shares its unpaired electron (remember that chlorine atoms have seven electrons in their outer shell) to form a covalent bond as shown. The "dot and cross" representation also shows the paired electrons. Each chlorine atom believes that it has the full octet because of the shared pair, and the energy required to break one mole of these bonds is quite considerably lower at 242 kJ per mole.
The energy "stored" in a chemical bond is often regarded as "chemical potential energy" which seems to be a mishmash between chemistry and physics. When a chemical reaction takes place, the electrons involved (which are already at a particular energy level) will try to move into a state of even lower energy if they can by forming more stable, less energetic bonds. If this happens there will be a certain amount of "extra" energy left over which will be released. In this sort of case the release of energy is characteristic of an exergonic reaction.
At GCSE level all you really need to know is that in a chemical reaction, if the energy of the products is lower than the energy of the reactants will be release of energy (usually, but not always heat energy) and this will be regarded as exothermic. If the energy of the products is higher than that of the reactants, the reaction will have taken (usually thermal) energy from the surroundings and this would be regarded as an endothermic reaction.
When you are carrying out calculations involving bond energies, remember that if it takes "x" kilojoules per mole to break a particular bond, then when that particular bond is formed it will release "x" kilojoules per mole. For example a carbon to hydrogen single bond C-H is a bond energy of 413 kJ per mole which means that it takes this amount of energy to break one mole (remember, this is the Avogadro number) of the bonds, but when one mole of these bonds are formed, 413 kJ per mole of energy will be released.
Q. Calculate the energy required to break the bonds in 11g Carbon Dioxide.
A. The Relative Molecular Mass of Carbon Dioxide is 44 therefore we are dealing with 0.25 mol of the gas. The structural formula for Carbon Dioxide is this:

This tells us that each MOLECULE contains 2 C=O bonds, and the bond energy (enthalpy) for this type of bond in Carbon Dioxide is 799 kJmol-1
To break 0.25 mol of CO2 bonds will need:

>> Questions <<