The Mole
The mole is a concept which is on the higher tier paper, this is not surprising given the fact that it is also one of the hardest concepts for students to grasp. At the end of the day the mole is simply a number, albeit it is a very big number.
Yes, quite predictably I usually choose a little rodent cartoon to represent this particular topic, almost certainly the first thing anybody would think of, but the mole in the context of chemistry and science is a completely different thing.
A “mole” of a substance (atoms or molecules) is the molecular weight of that substance (atoms or molecules) expressed in grams. So, for example a mole of the element carbon would be 12 g because the accepted relative atomic mass of that element is 12.
This is not confined to atoms, compounds can also be described as having a molarity based on their relative molecular mass so for example the molecule of water (H2O) with a molecular mass of 18 would have a mass of 18 g per mole, similarly sulphuric acid (H2S04) would have a mass of 98.1 g per mole.
This can lead to some quite interesting mathematics because we now bring into the discussion the Avogadro number or Avogadro constant which is this:

When we talk about 1000, we write it as it is shown... 1 followed by three zeros.
When we move on to larger numbers such as millions billions and trillions, the situation gets a little bit harder to manage and this is only because we are not used to dealing with such large numbers, trillions rarely feature in everyday conversation and use so it is not surprising that many students (and probably many adults) would not be able to write down the number if they were asked to.
|
10 |
1 |
TEN |
|
100 |
2 |
HUNDRED |
|
1 000 |
3 |
THOUSAND |
|
1 000 000 |
6 |
MILLION |
|
1 000 000 000 |
9 |
BILLION |
|
1 000 000 000 000 |
12 |
TRILLION |
|
1 000 000 000 000 000 |
15 |
QUADRILLION |
|
1 000 000 000 000 000 000 |
18 |
QUINTILLION |
|
1 000 000 000 000 000 000 000 |
21 |
SEXTILLION |
|
602 000 000 000 000 000 000 000 |
MOLE |
MOLE (AVOGADRO NUMBER) |
|
1 000 000 000 000 000 000 000 000 |
24 |
SEPTILLION |
|
1 000 000 000 000 000 000 000 000 000 |
27 |
OCTILLION |
As you can see, numbers up to perhaps 1,000,000,000 are reasonably familiar, although even numbers as relatively small as 1,000,000,000 can cause problems depending on the numbering system you're following. In the United States 1,000,000,000 is one followed by 9 zeros but in the United Kingdom 1,000,000,000 can also be regarded as 1,000,000 million which would be one followed by 12 zeros. We have to decide which representation we are going to adopt, and I believe that the commonest definition in use is that from the United States, sufferer purposes 1,000,000,000 will be one followed by 9 zeros.
The mole is simply a number, as you can see where it lies between sextillion and septillion the mole is approximately 602 sextillion. Just a number, but a very big one!
This large number is known as the Avogadro constant all the Avogadro number and a mole of particles, indeed a mole of anything contains exactly this number of particles. So a mole of marbles, a mole of cars, a mole of blackbirds or even a mole of moles would contain this many of each particular entity. In chemistry we extend the definition to state that:
"A mole of a substance is that amount of the substance that contains the Avogadro number of particles. This is the same as the number of grams of the element or compound as is represented by its relative atomic mass or relative molecular mass"
To try to demystify this definition a little bit, take the element carbon (deliberately chosen because at the moment this is the baseline) the relative atomic mass of which is 12.011. Often we rounded to the nearest whole number so we will consider 12 for our purposes. A mole of carbon will therefore contain 12 g.
Q. How many atoms in 0.023 moles of xenon gas?
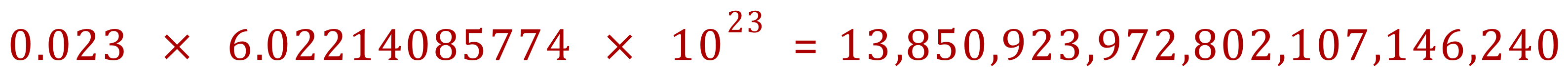
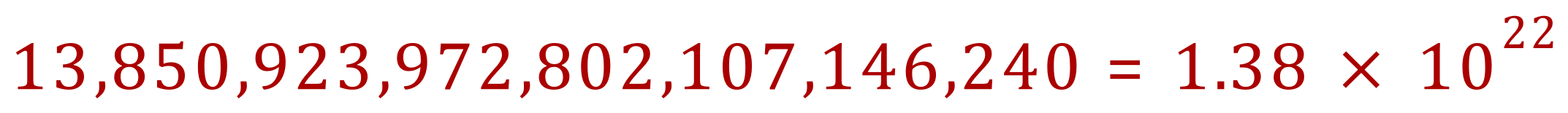
(Obviously this cannot be quoted as exact)
And just out of interest, as the relative atomic mass of xenon is 131.3 we can see that 0.023 moles would weigh approximately 3.02 g
>> Questions <<
Back To >> Energy Changes In Reactions <<