Calculating Energy Changes in Reactions
Q1. Hydrogen gas reacts with Chlorine gas to produce Hydrogen Chloride. From the balanced equation given, and the bond energies, calculate the energy of the reaction and state whether or not it is exothermic or endothermic.
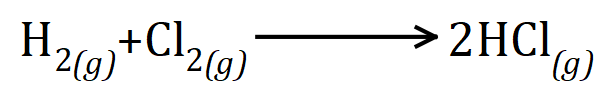
|
Bond |
Bond energy |
|
H−H |
436 kJ mol-1 |
|
Cl−Cl |
243 kJ mol-1 |
|
H−Cl |
432 kJ mol-1 |
Q2. Hydrogen gas reacts with Oxygen gas to form Water according to the following equation:
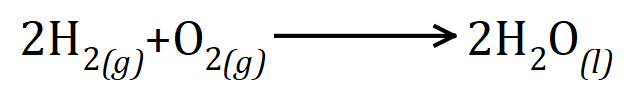
From this information and the bond energies provided, calculate the energy change for the reaction.
|
Bond |
Bond energy |
|
H−H |
436 kJ mol-1 |
|
O=O |
498 kJ mol-1 |
|
O-H |
464 kJ mol-1 |
Q3. The alcohol Methanol burns in excess oxygen (in air) completely to produce Carbon Dioxide and Water according to the following equation:

|
Bond |
Bond energy |
|
C-O |
358 kJ mol-1 |
|
C=O |
805 kJ mol-1 |
|
O-H |
464 kJ mol-1 |
|
C-H |
413 kJ mol-1 |
|
O=O |
498 kJ mol-1 |
From this information and the table of bond energies provided, calculate the energy change for the combustion of Methanol. (NB: this is a simple reworking of one of the example questions but see if you can still remember how to do it).
Q4. Ethene (ethylene) reacts with bromine as shown in the equation below.
(This is in fact the standard test for Alkenes and unsaturation in organic molecules.)
The overall energy change for the reaction is -122 kJ per mole. Given this information and the remaining bond energies, calculate the bond energy of the Carbon-Bromine bond.
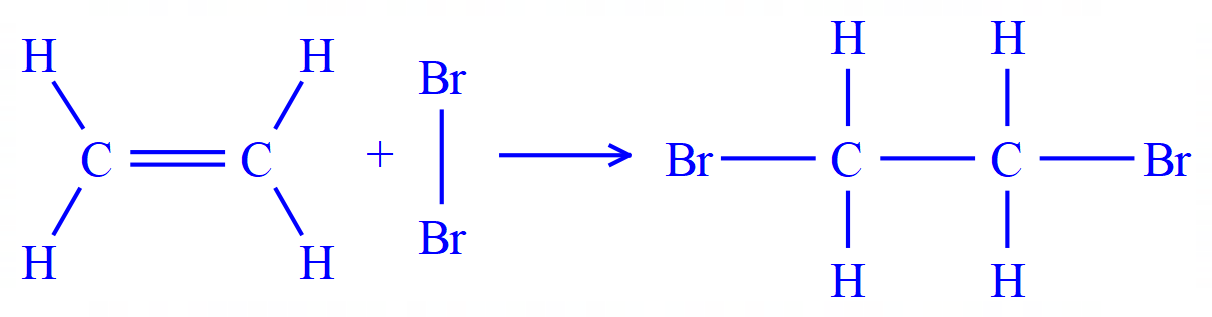
|
Bond |
Bond energy |
|
C-H |
413 kJ mol-1 |
|
C=C |
612 kJ mol-1 |
|
Br-Br |
193 kJ mol-1 |
|
C-C |
348 kJ mol-1 |
Q5. There are a number of methods that we can use to make Carbon Tetrachloride, one of them is by the chlorination of Methane according to the following equation:

|
Bond |
Bond energy |
|
C-H |
413 kJ mol-1 |
|
C-Cl |
327 kJ mol-1 |
|
Cl-Cl |
242 kJ mol-1 |
|
H-Cl |
431 kJ mol-1 |
Use this information, and the bond energy values provided to calculate the overall enthalpy change (energy change) for the reaction. State if this is an exothermic or an endothermic reaction.
Go To >> Solutions <<
Back To >> Energy Changes in Reactions <<
Reference >> Bond Energies <<