Atom Economy
Q1. Hydrogen can be manufactured by reacting methane with steam:
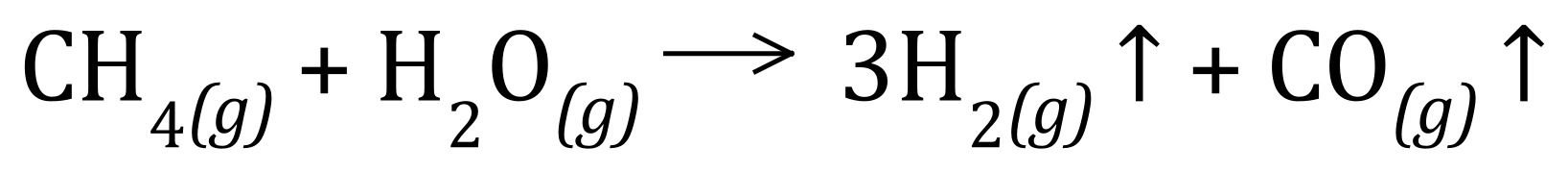
Calculate the atom economy for the reaction. You are given the Relative Atomic Masses of the elements as H = 1, C = 12, O = 16.
Q2. Ethanol, CH3CH2OH, can be produced by the fermentation of glucose, C6H12O6:
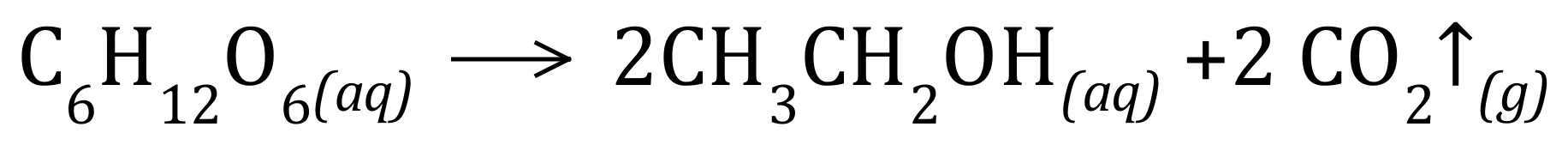
Calculate the atom economy for the reaction. You are given the Relative Atomic Masses of the elements as H = 1, C = 12, O = 16.
Q3. Nickel can be produced by the reduction of Nickel Oxide with Carbon, producing Nickel metal and Carbon Monoxide. Given the reaction for the equation:
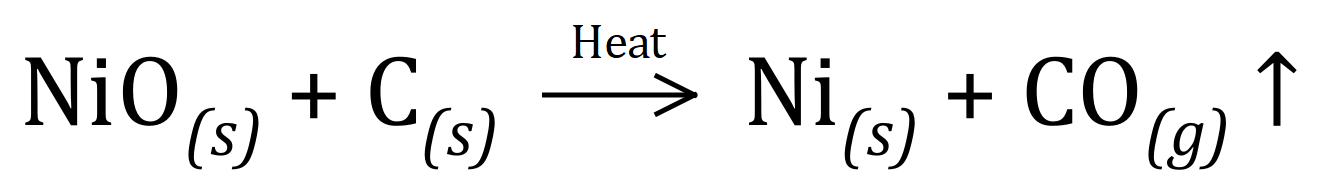
And the relative atomic / molecular masses needed (Ni = 59, C = 12, O = 16), calculate the Atom Economy of this reaction.
Q4. Magnesium metal can be produced by reacting Magnesium Oxide with Silicon according to the following reaction:
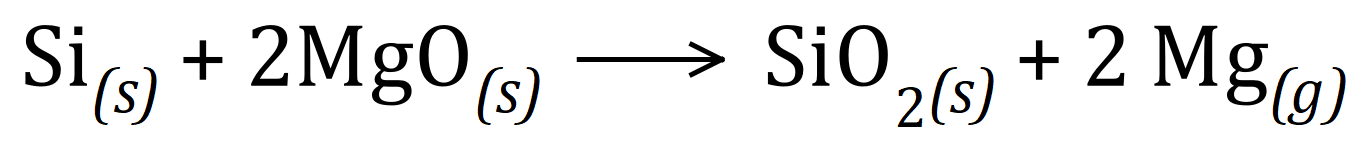
Given the RMM and RAM of the elements and compounds concerned, what is the Atom Economy of this reaction? (Si = 28, Mg = 24, O = 16)
Go To >> Solutions <<
Back To >> Atom Economy <<