Atom Economy
We know that from the law of conservation of mass, we cannot gain or lose any atoms during a chemical reaction. In other words if for example a chemical reaction starts off with 3 carbon atoms then in some way shape or form it must end with 3 carbon atoms, they may have course be in different compounds but they will still exist. In school level chemistry we aren't too worried about the economics of the reactions, school science requires that we at least try to arrive at the right result but in industrial processes which can be quite costly, the economics of the process has to become forefront. We talk therefore in industry about "atom economy" which is the amount of atoms that end up forming useful products during the reaction. Of course the more reactant which turns into useful product the higher the atom economy and the better it is economically. So let us take a look at what exactly "atom economy" is.
Atom economy can be represented quite simplistically by a word equation:

Where RFM stands for the "relative formula mass" (you may sometimes see this referred to as the relative molecular mass) which is the sum of all of the atomic masses of the elements making up the compound, so for example if one of the compounds was sulphuric acid, the relative formula mass would be 98 which is the sum of H (twice) S (once) and O (4 times) making of course 98. Note that the atom economy is expressed as a percentage and only concerns the desired products, however it must include all reactants which go together to make those desired products as in many cases it will almost certainly be some waste product which will serve to reduce the atom economy.
Q. Calculate the atom economy in this rather expensive way of producing water, from the burning of propane gas in oxygen.
A. The first thing we need to do in any of these types of calculations is to establish the balanced chemical equation for the reaction:

Now we need to work out a few relative formula masses, given the relative atomic masses of the elements concerned:
C = 12, H = 1, O = 16
From this we can establish the relative formula mass of propane is 44, oxygen is 16 and on the left-hand side of the equation we have 10 of them so this is 160 total and the relative formula mass of our desired product, water is 18 but we have 4 moles of water produced therefore we are 4×18 which equals 72.
Atom economy for this reaction is therefore:
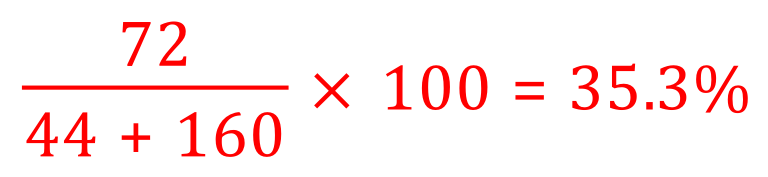
35.3% doesn't indicate a particularly lucrative way to make water, and I can't see that this would ever be done, but the calculation serves to show you how atom economy is arrived at.
Q. Calculate the atom economy of the reaction between magnesium oxide and hydrogen chloride/hydrochloric acid in the production of magnesium chloride and water, given that the required product is magnesium chloride.
Mg = 24, O = 16, H = 1, Cl = 35.5
A. As is always the case, first of all establish the balanced chemical equation for the reaction:

You are given the relative atomic masses of all of the elements necessary, so you can work out that the relative formula mass for magnesium oxide is 24+16 which equals 40, hydrochloric acid is 1 +35.5 which equals 36.5 and magnesium chloride is 24+ (35.5×2) which equals 95. We are told that the desired product is the magnesium chloride, the reactants are magnesium oxide (40) and hydrochloric acid (36.5) however you should note that 2 moles of hydrochloric acid are required for each mole of magnesium oxide so the sum of the relative formula masses of the reactants becomes 40+ (2×36.5) which equals 113.
Now we can proceed to the calculation:
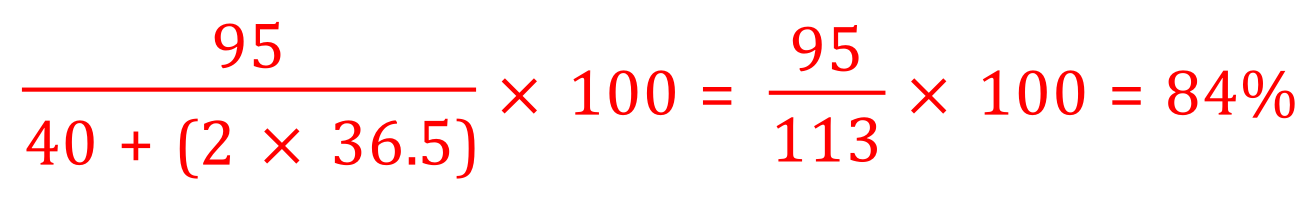
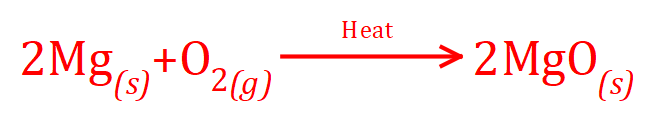
Q. A student burns a strip of magnesium in a hot Bunsen burner flame in excess oxygen to produce magnesium oxide. What is the atom economy of this reaction?
A. First of all, as you've probably expected, write out of the balanced chemical equation for the reaction:
Stop and think a little bit, where do you think that this question is going? We have one product which is the desired product? What can you conclude from this?
If we have one product and it is the desired product, then we must have an atom economy of 100% as we are creating no waste (in our first experiment regarding the production of water from propane we created a waste product, carbon dioxide and in the second example we created water as a waste product. In this case there are no waste products and so if there is no waste, the economy must be 100% but let's prove it by carrying out the calculations:
Mg = 24, O = 16
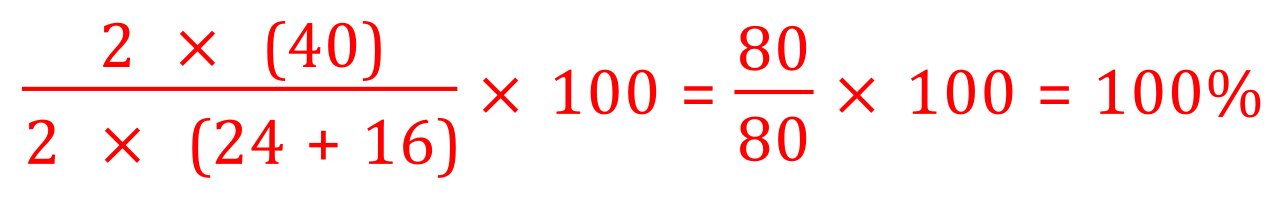
As the law of conservation of mass quite rightly tells us, no atoms are created or lost during the chemical reaction, which leads us to the result that we have just proven.
Q. There are a number of ways to produce carbon dioxide:
(a) Dissolving sodium hydrogen carbonate in dilute hydrochloric acid.
(b) Thermal decomposition of calcium carbonate.
(c) Combustion of methane in oxygen.
Given the relative atomic masses of Na = 23, H = 1, O = 16, C = 12, Ca = 40 Cl = 35.5 calculate the atom economies of the different methods given.
A. As always, we need to write out the balanced chemical reactions for the 3 methods given:
(a)

(b)
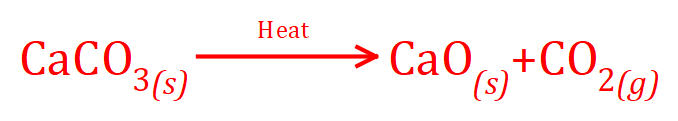
(c)

All 3 of these reactions produce an amount of carbon dioxide, what we need to do now is to apply the equation for atom economy to see which would be the most efficient in terms of percentages.
For the reaction between sodium hydrogen carbonate and hydrochloric acid, we first of all work out the relative formula masses of the reactants and the desired product carbon dioxide.
NaHCO3 = 23+1+12+(16 x 3) = 84, HCl = 1 + 35.5 = 36.5, CO2 = 12 + (16 x 2) = 44.
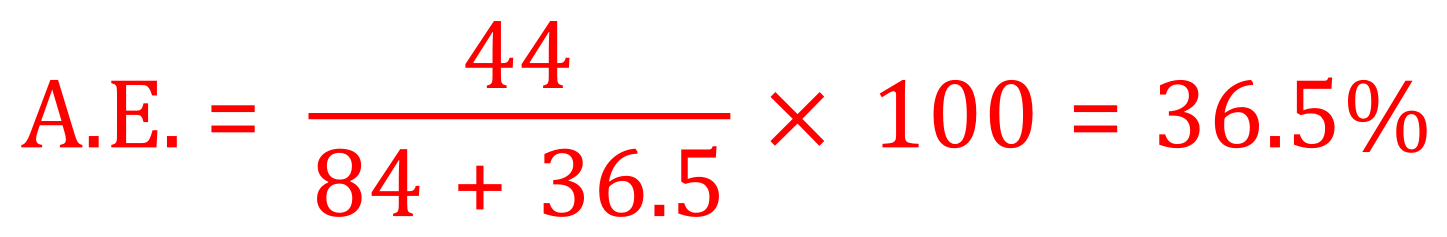
For the thermal decomposition of calcium carbonate:
CaCO3 = 40+12+(16 x 3) = 100, CO2 = 44 as before.
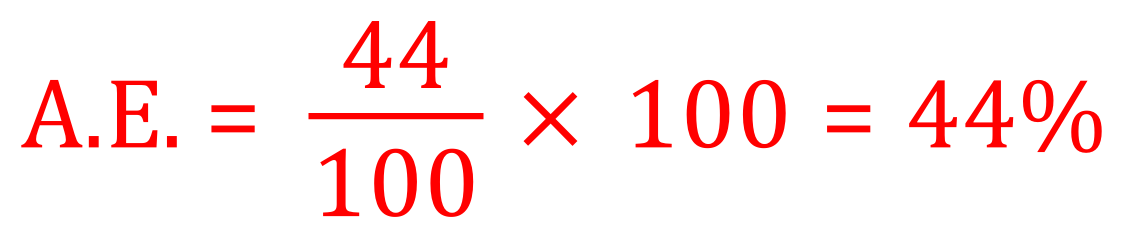
And finally for the combustion of methane in oxygen:
CH4 = 12 + (1 x 4) = 16, 2O2 = 2 x (16 x 2) = 64 and CO2 = 44 as before.
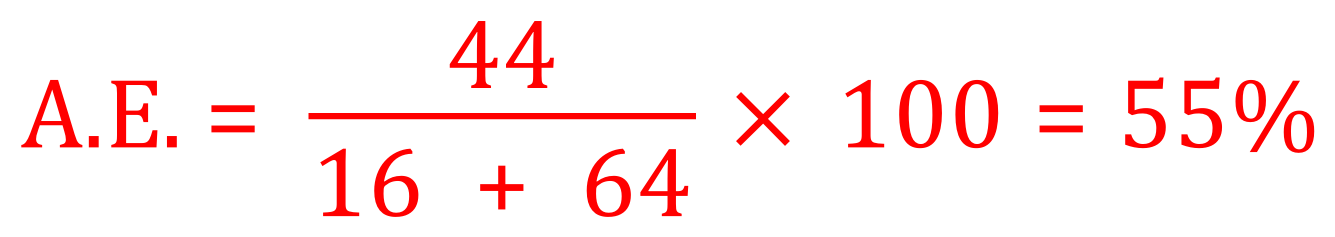
It is interesting to see that what would appear to be the most wasteful reaction (although burning methane does provide heat) also appears to be the most economical in terms of production of carbon dioxide, the downside of this of course is that carbon dioxide is a greenhouse gas and combustion of methane appears to be one of the best ways to make it! The main industrial processes used to create carbon dioxide often rely on the capture of carbon dioxide when it is produced as a waste product however in the production of ammonia, natural gas is burnt to separate the carbon and hydrogen, the hydrogen combines with nitrogen to produce ammonia and the carbon combines with oxygen to produce carbon dioxide. This would appear to be one of the main methods to produce the gas.
Q. A scientist is comparing 2 reactions to make potassium hydroxide:


(a) In the case of both methods, calculate the atom economy for the production of potassium hydroxide, given the relative atomic masses of H=1, O=16, Cl=35.5, K=39 and Ca=40
A. In the case of the first reaction:

The relative formula mass of potassium hydroxide is (39+16+1) which is 56 and because we make two moles of it in a reaction we deal with the number 112.
The relative formula mass of potassium chloride is 39+35.5=74.5 and the relative formula mass of water is 18, but since we use two moles of each compound in the reaction we double these values up to 149 and 36 respectively. If we had 149 and 36 together we arrive at 185, at calculation for the atom economy therefore becomes:

B. In the case of the second reaction between calcium hydroxide and potassium carbonate we can see from the balanced equation that one mole of calcium hydroxide will react with one mole of potassium carbonate to produce one mole of calcium carbonate and 2 moles of potassium hydroxide.
One mole of calcium hydroxide has a relative formula mass of 40+ 2*(16+1) = 74 and one mole of potassium carbonate has a relative formula mass of (2*39)+12+(3*16) = 78+12+48 = 138. The combined relative formula masses of our reactants is therefore 74+138 which equals 212. The reaction produces two moles of potassium hydroxide which has a relative formula mass, as we have seen before, of 56, but as we are producing 2 moles of it we double this up to 112.
The calculation for the atom economy is therefore:

>> Questions <<