Percentage Yield
We talked previously about atom economy, and the higher the atom economy the better (no surprises, economically) the reaction is. We have also seen however that in the production of a particular desired product we often produce by-products which may or may not be useful in their own right.
We now move on to another aspect of "chemical economics" if we can call it that, and that is the subject of percentage yield. Theoretically and according to the law of conservation of mass if we react two components A and B to produce C we should in theory obtain a full 100% yield of product C, although this is pretty much never the case. We investigate the "theoretical yield" arithmetically, we investigate the actual yield often by experimentation/investigation (i.e. actually making the stuff) and then calculate the percentage yield again using simple arithmetic.
We calculate the theoretical yield from the balanced chemical equation so once again it is important that you understand how to produce equations and make sure that they are balanced as it is possible that in an examination question you might be asked to produce the balanced equation for the first few marks and then go on to use the result of that calculation in a percentage yield evaluation for the remainder of the marks of the question.
Consider the following chemical reaction:

(a) This is a decomposition reaction between lithium hydroxide and potassium chloride, the products of which are lithium chloride and potassium hydroxide. In this reaction we react 20 g of lithium hydroxide with an excess of potassium chloride, what would be the theoretical yield of lithium chloride?
Let's take a look at some relative formula masses, first of all let us establish the relative atomic masses of the elements concerned:
Li = 7, O = 16, H = 1, K = 39, Cl = 35.5
From the reaction we can see that 1 mole of lithium hydroxide would react with 1 mole of potassium chloride to produce 1 mole of lithium chloride and 1 mole of potassium hydroxide. A fairly straightforward one to one ratio displacement/decomposition reaction.
The relative formula mass of lithium hydroxide is 7 + 16 + 1 = 24
The relative formula mass of potassium chloride is 39 + 35.5 = 74.5
The relative formula mass of lithium chloride is 7 + 35.5 = 42.5
The relative formula mass of potassium hydroxide is 39 + 16 + 1 = 56
We can therefore state that theoretically, 24 g of lithium hydroxide reacted with 74.5 g of potassium chloride would in theory produce 42.5 g of lithium chloride and 56 g of potassium hydroxide. These are the theoretical yields, the actual yields will be different.
(b) The question tells us in fact that 20/24 (0.8333...) mole of lithium hydroxide is used and therefore this should lead to the production of 20/24 (0.8333...) mole of lithium chloride but in fact we only produce 6 g of lithium chloride. The percentage yield is therefore calculated thus:

As you've probably already surmised, this is a pretty low percentage yield.
Calculating percentage yield is relatively straightforward:
Q. A student reacts 25 g of copper oxide with excess sulphuric acid to produce aqueous copper sulphate solution and water. The student wishes to evaporate off the water and crystallise the copper sulphate.
(a) Calculate the maximum theoretical yield of copper sulphate crystals that the student could produce with this amount of copper oxide
(b) The actual amount of copper sulphate that the student manages to crystallise is 23.6g, calculate the percentage yield.
A.
(a) The first thing that we should consider is to create the balanced chemical equation for the reaction between copper oxide and sulphuric acid.
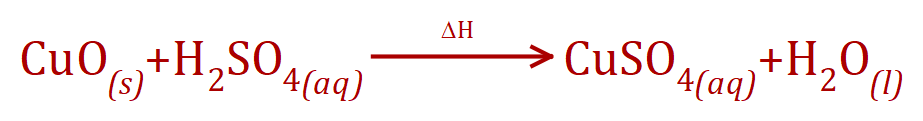
Using the symbol "Delta H" is just a shorthand way of showing that the reaction requires gentle application of heat to ensure the dissolution of the copper oxide. This reaction would normally take place in a Pyrex beaker on a tripod and gauze with a gentle Bunsen burner flame. We can see from the equation that this is a simple one-to-one displacement reaction between copper oxide and sulphuric acid, in that 1 mole of copper oxide will react with 1 mole of sulphuric acid to produce 1 mole of copper sulphate and a mole of water.
The relative atomic masses of the elements concern are H = 1, Cu = 63.5, O = 16, S = 32. Therefore we can establish the relative formula masses of the compounds reacted viz:
CuO = 63.5 + 16 = 79.5
H2SO4 = (2 x 1) + 32 + (4 x 16) = 98
CuSO4.5H2O = 63.5 + 32 + (4 x 16) + 5 x (18) = 249.5
H2O = (2 x1) + 16 = 18
It should be noted that blue copper sulphate crystals are examples of the copper sulphate pentahydrate, therefore each copper and sulphate ion pair will be accompanied by 5 molecules of water adding a further 90 units to the relative formula mass.
From the balanced equation we can therefore establish that 79.5 g of copper oxide would react with 98 g of sulphuric acid to produce 249.5 g of copper sulphate and 18 g of water.We are told in the question the student uses 25 g of copper oxide which is 25÷79.5 = 0.31 moles, So the theoretical maximum mass of copper sulphate which could be produced from these amounts is 0.31 moles or 0.31×249.5 = 78.5 g.
(b) In the 2nd part of the question we are told that the student actually crystallises 23.6 g where the expected theoretical maximum is 78.5, therefore the percentage yield is 23.6÷78.5 x 100 = 30%
Q. A scientist working in a small scale laboratory wishes to produce Aspirin from the reaction of Salicylic Acid and Ethanoic Anhydride in the following reaction which produces Aspirin and Ethanoic Acid as a byproduct.
The reaction is as shown below:
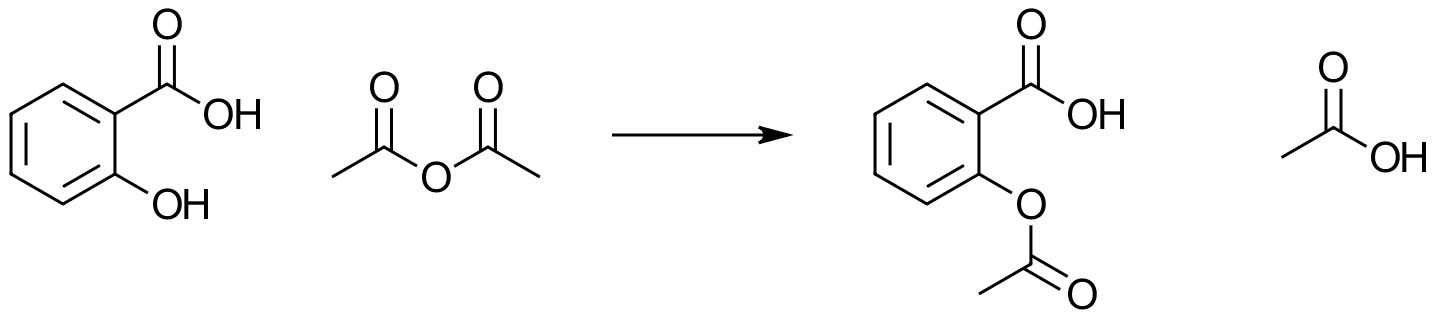
The relative formula masses of the compounds involved are as given:
Salicylic Acid (138.12) Ethanoic Anhydride (102.09) Ethanoic Acid (60.05) and the product Aspirin (Acetyl Salicylic Acid) (180.158). The scientist started off with 6.14 g of salicylic acid and ultimately produced 2.64 g of the crude product. Calculate the theoretical yield and actual yield from these data.
A. Although the equation appears to be quite complicated it is in fact a simple one-to-one reaction where 1 mole of salicylic acid requires 1 mole of ethanoic anhydride to produce 1 mole of aspirin and 1 mole of the by product ethanoic acid. Using the relative formula masses therefore tells us that 138.12 g of salicylic acid should yield 180.158 g of aspirin. As the scientist used 6.14 g of salicylic acid this works out as:
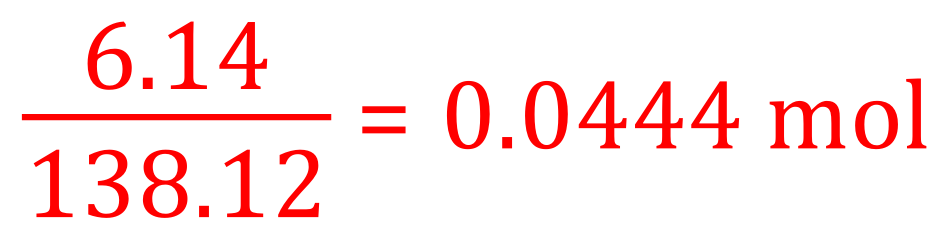
The theoretical yield of aspirin should therefore be 0.0444 moles which evaluates to:

The scientist actually created only 2.64 g of the crude product, which works out as:
