Calculating Energy Changes in Reactions
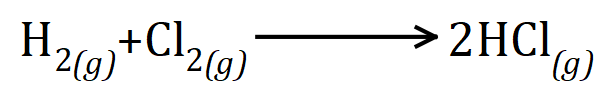
Q1. Hydrogen gas reacts with Chlorine gas to produce Hydrogen Chloride. From the balanced equation given, and the bond energies, calculate the energy of the reaction and state whether or not it is exothermic or endothermic.
|
Bond |
Bond energy |
|
H−H |
436 kJ mol-1 |
|
Cl−Cl |
243 kJ mol-1 |
|
H−Cl |
432 kJ mol-1 |
It can sometimes help during this sort of question to visualise the numbers of bonds being broken and the number of bonds being formed by rewriting the balanced equation using the displayed formulae of the elements/compounds concerned.
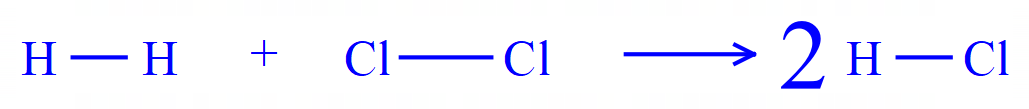
Showing the equation this way gives you a clearer picture of the bonds that are going to be broken and the bonds that are going to be formed, and the respective numbers of each. From the information as we have it now we can see that one H-H bond is going to be broken and one Cl-Cl is also going to be broken, but the result is that two H-Cl bonds will be formed.
On the left-hand side and we are going to require to put in (1×436) + (1×243) = 679 kJ/mol but the energy that we will receive after the reaction (on the right-hand side) will be (2×432) = 864 kJ/mol.
The energy change during the reaction (usually shown as "Delta H") will be the difference between the energy supplied and the energy obtained during the reaction, another way of putting this is energy in - energy out.
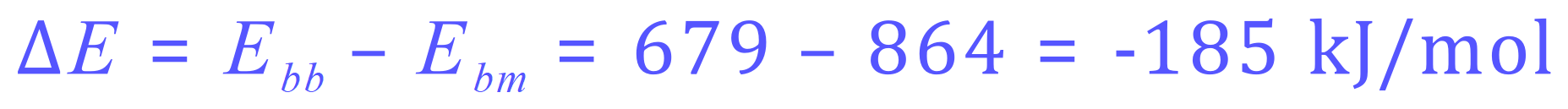
We can see that the reaction is exothermic releasing 185 kJ per mole.
Q2. Hydrogen gas reacts with Oxygen gas to form Water according to the following equation:
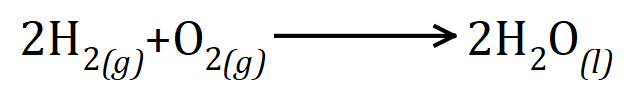
From this information and the bond energies provided, calculate the energy change for the reaction.
|
Bond |
Bond energy |
|
H−H |
436 kJ mol-1 |
|
O=O |
498 kJ mol-1 |
|
O-H |
464 kJ mol-1 |
Remember, if it helps then re-state the balanced equation as a "displayed" equation so that you can visualise the number and type of bonds being broken and formed.
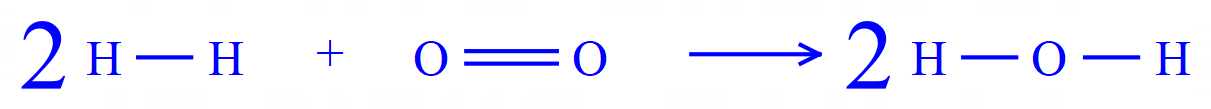
In this example:
Bonds broken (Ebb) = (2x436) + (1 x 498) = 1370 kJ/mol
Bonds made (Ebm) = (4 x 464) = 1856 kJ/mol
Note that you have TWO water molecules, BUT there are TWO O - H bonds in each, so FOUR in total are being made.
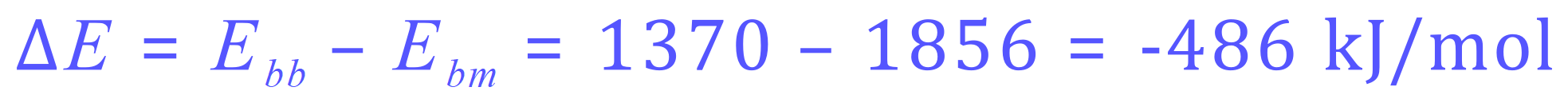
Once again a strongly exothermic reaction releasing 486 kJ/mol of energy to the system (environment).
Q3. The alcohol Methanol burns in excess oxygen (in air) completely to produce Carbon Dioxide and Water according to the following equation. (NB: this is a simple reworking of one of the example questions but see if you can still remember how to do it):

|
Bond |
Bond energy |
|
C-O |
358 kJ mol-1 |
|
C=O |
805 kJ mol-1 |
|
O-H |
464 kJ mol-1 |
|
C-H |
413 kJ mol-1 |
|
O=O |
498 kJ mol-1 |
From this information and the table of bond energies provided, calculate the energy change for the combustion of Methanol.
Again, it is useful to show the equation in its displayed form so that you can actually see the bonds concerned (those being broken and of course those being made) and the relative numbers of each. This makes the arithmetic easier.
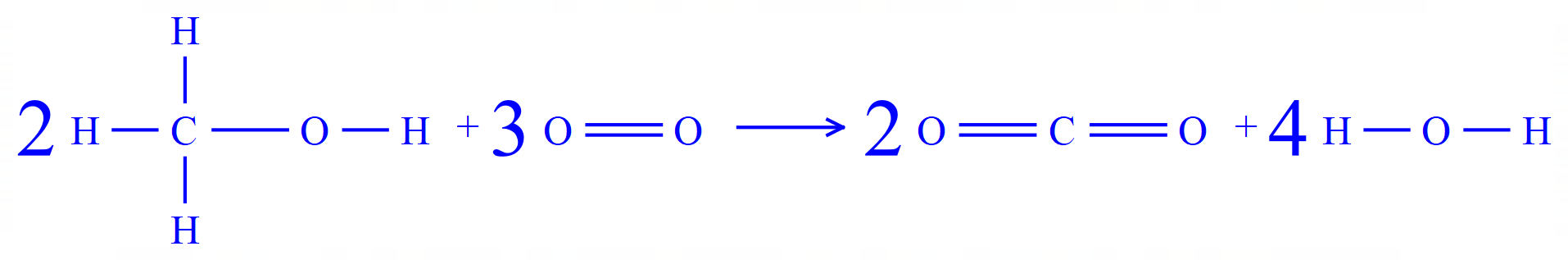
Now we need to count the numbers of the different types of bond and multiply them by the appropriate bond energy from the table.

This time I have entered the data into a small table. As the reactants and products become a little bit more complicated, the number of bonds made and the number of bonds broken will usually increase. Tabulating your data in this way give you a way to keep track on the numbers you're producing, and helps to ensure that you come to the right result at the end.
Finally:
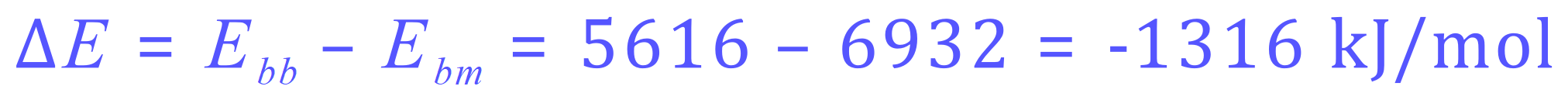
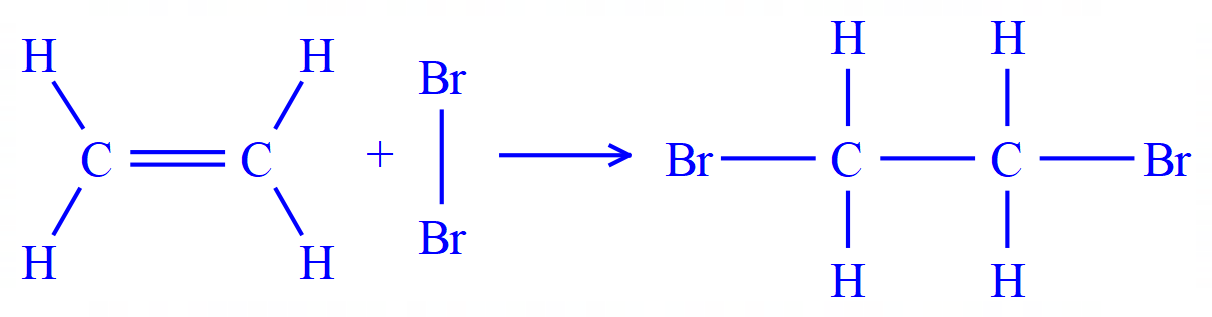
Q4. Ethene (ethylene) reacts with bromine as shown in the equation below.
(This is in fact the standard test for Alkenes and unsaturation in organic molecules.)
The overall energy change for the reaction is -122 kJ per mole. Given this information and the remaining bond energies, calculate the bond energy of the Carbon-Bromine bond.
|
Bond |
Bond energy |
|
C-H |
413 kJ mol-1 |
|
C=C |
612 kJ mol-1 |
|
Br-Br |
193 kJ mol-1 |
|
C-C |
348 kJ mol-1 |
This is a slightly different question. This time you're not given all of the bond energies and asked to work out an overall result, you are given the overall result and all but one of the bond energies, and this is your task (to find the missing one).
Let's take a look at the information we've got, first of all we have the displayed version of the reaction involved so we can work out the bonds that we are going to break and the bonds we are going to form quite easily. Again I would advise you to tabulate your results as in the previous question.
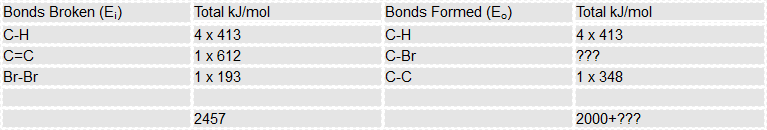
This time we know "Delta E" so our calculation can be rewritten in a slightly different way:

Look at the input energy we can see that we have all of the values concerned, but the output energy leaves us with a problem because we do not know the strength of the carbon bromine bond, in the table have called it "???", but within the calculation I will call it 'x'.
Rearranging what we know so far....
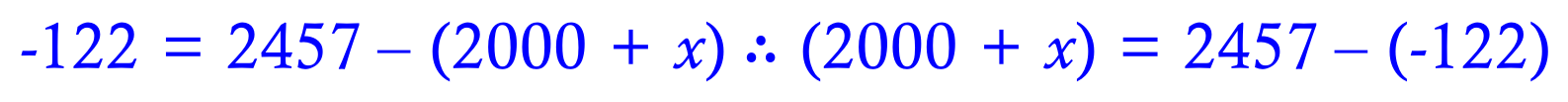
You may need to study this rearrangement for a short while to make sure that you understand where it came from.
Ready to continue? ... OK
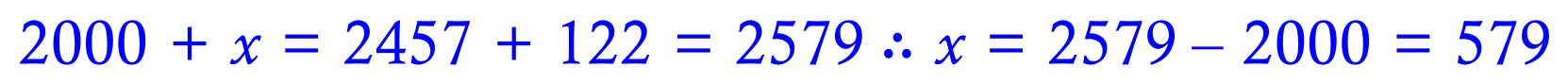
This value of 579 kJ per mole does of course refer to two Carbon-Bromine bonds, because the reaction produces two.
The bond energy of a single Carbon-Bromine bond is therefore 289.5 kJ per mole.
Q5. There are a number of methods that we can use to make Carbon Tetrachloride, one of them is by the chlorination of Methane according to the following equation:

|
Bond |
Bond energy |
|
C-H |
413 kJ mol-1 |
|
C-Cl |
327 kJ mol-1 |
|
Cl-Cl |
242 kJ mol-1 |
|
H-Cl |
431 kJ mol-1 |
Use this information, and the bond energy values provided to calculate the overall enthalpy change (energy change) for the reaction. State if this is an exothermic or an endothermic reaction.
Once again, rewrite the equation for the reaction in terms of the displayed formula for each of the products and reactants.
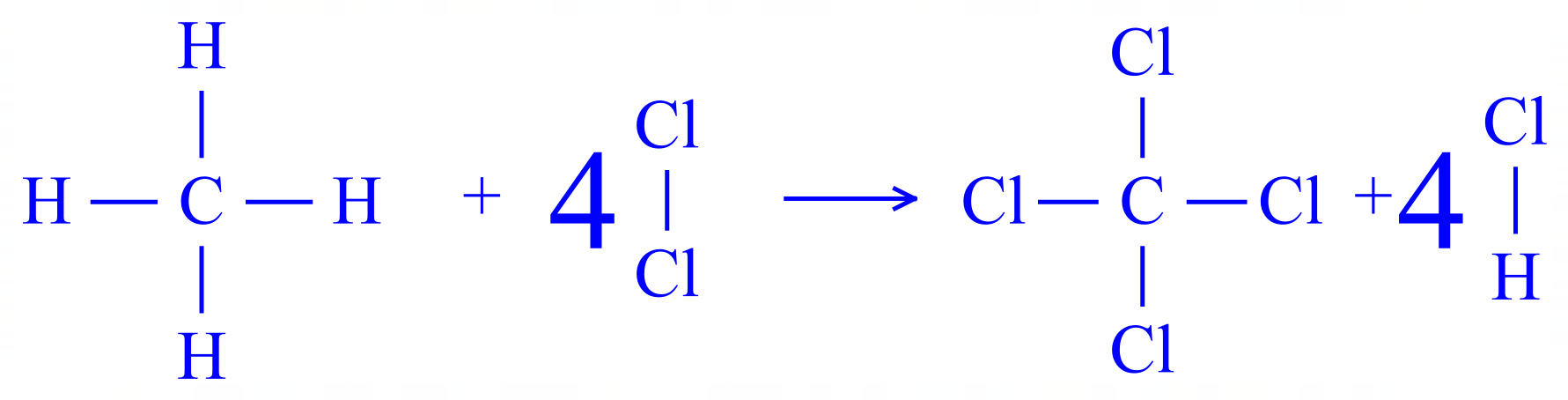
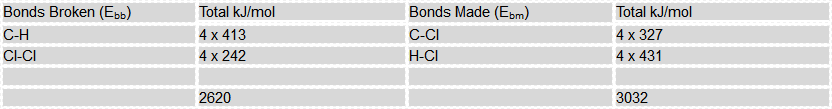
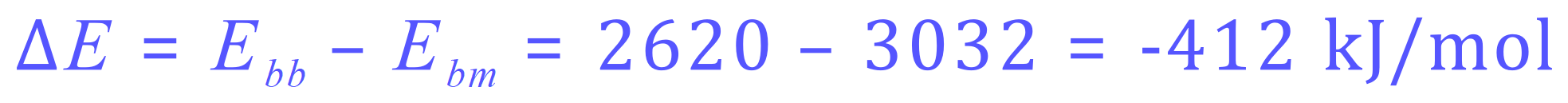
Once again, a considerably exothermic reaction as 412 kJ/mol energy is released to the environment.
Go To >> Questions <<
Back To >> Energy Changes in Reactions <<
Reference>> Bond Energies <<