Balancing Chemical Equations
1.


2.


3.

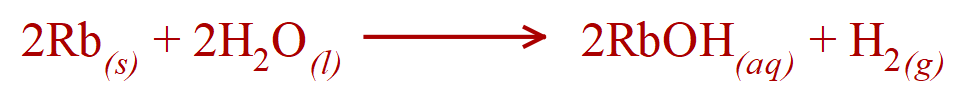
4.


5.


6.
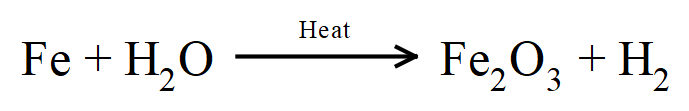

7.


8.


9.


10.


11.


12.


13.


14.


15.


16.


17.


18.
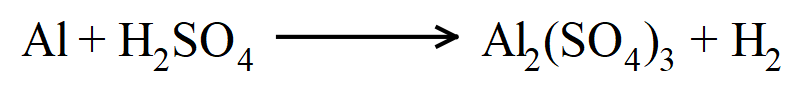

19.
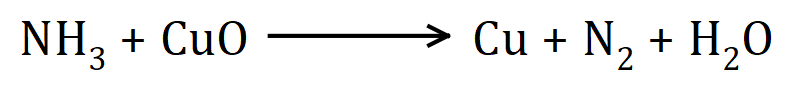

20.

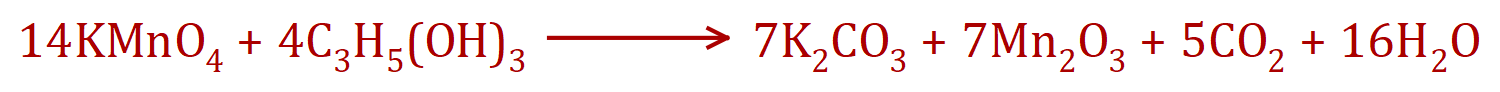
Note that in the case of the last equation, number 20, this is the equation of a fascinating experiment which would be conducted in a fume cupboard, by the teacher. Potassium (VII) Manganate (used to be called Potassium Permanganate) reacts with Glycerol quite violently producing sparks, lots of smoke and a general purple colouration however the reaction does take quite some time to start.
From the equation you can see that the products are Potassium Carbonate, Manganese (III) Oxide, Carbon Dioxide and Water. The equation is horrendous and you would not be asked in any examination to try to balance this sort of thing as you will almost certainly pass through many stages and it would take a very long time indeed.
Back to >> Questions <<
Back to >> Balancing Chemical Equations <<