Halogen Displacement Reactions
Q. State whether a displacement reaction would occur between the following pairs of halogen solutions, if you think there will be no reaction, explain why. If you think there will be a reaction, explain why and support your answer with balanced equations showing the reaction.
a. Chlorine water and Sodium Bromide solution
Yes - if you pass Chlorine water through a solution of Sodium Bromide, Bromine will be displaced in solution which will turn orange brown. this is because Chlorine is more reactive than Bromine and will displace Bromine from solution forming a solution of Sodium Chloride with Bromine displaced into it.
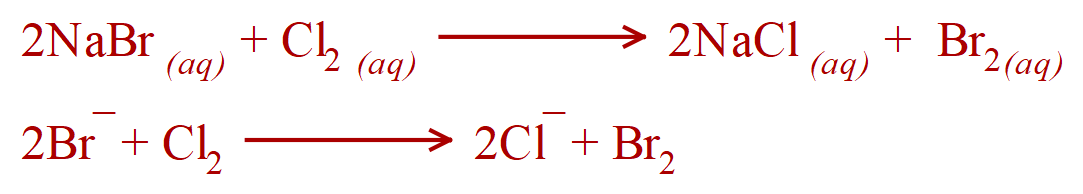
b. Iodine solution and Lithium Fluoride solution.
No - Iodine is far less reactive than Fluorine therefore there will be no effect on the Fluoride ions already in solution.
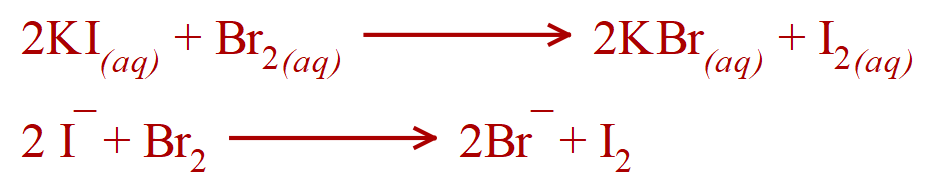
c. Bromine water and Potassium Iodide solution.
Yes - Bromine is more reactive than Iodine and will therefore displace Iodide ions from solution as Iodine (the Iodide ions are said to have been "oxidised", with Bromine atoms "reduced" to Bromide ions).
Back to >> Questions <<
Back to >> Group 7 - The Halogens <<