Group 7/17 - The Halogens
The halogens range from Fluorine (atomic number 9) to Tennessine (atomic number 117) and are characterised by the fact that they all contain seven electrons in their outer shell. We know that atoms tend to like the complete octet and so to be so close to completion is a contributory factor in the reactivity of this particular group of elements.
|
|
|
|
|
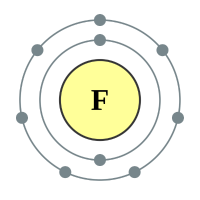
Fluorine |
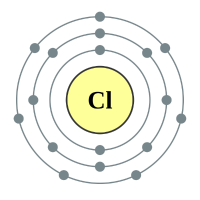
Chlorine |
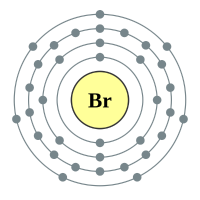
Bromine |
|
2 shells - 9 electrons - [2,7] |
3 shells - 17 electrons - [2,8,7] |
4 shells - 35 electrons - [2,8,18,7] |
|
|
|
|
|
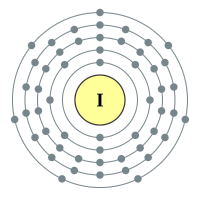
Iodine |
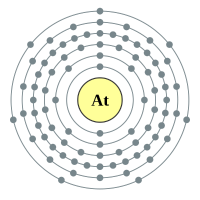
Astatine |
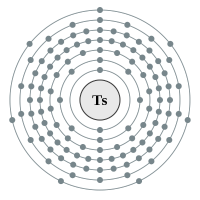
Tennessine |
|
5 shells - 53 electrons - [2,8,18,18,7] |
6 shells - 85 electrons - [2,8,18,32,18,7] |
7 shells - 117 electrons - [2,8,18,32,32,18,7] |
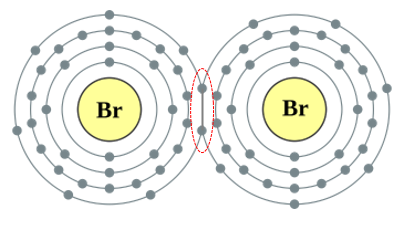
The Halogens aren't a particularly friendly group. The first four are highly poisonous and there isn't much known about the other two at this moment. The halogens will form ionic compounds with metals by accepting a donated electron. The halogens (certainly the ones we know about) exists in the diatomic state because they do in fact share one of the outermost electrons, which convinces each atom in the molecule that it has the eight electrons in its outer shell required to satisfy the octet rule.
|
|
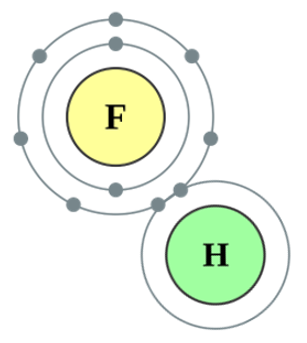
With other non-metals the halogens will form covalent compounds, for example with Hydrogen, the outermost electron (indeed the only electron!) will be shared by the Hydrogen atom with the halogen, the halogen will in return share one of its outermost seven electrons. As a result of the sharing, Hydrogen now thinks it has two electrons (a full outer shell) and the halogen believes that it too has a full outer shell, everybody's happy!
What I have tried to do above is to give you an indication of the electronic configurations of the respective atoms, leading to (in red) a suggestion as to how they see themselves after the bond has been formed. Since the inner shell of an atom can only contain two electrons then Hydrogen is indeed satisfied that it now has a full outer shell. |
The fluorine atom is sharing the hydrogen atom's single electron, as a result of which the gap in its outer shell is now complete. The fluorine atom here (which would technically become a fluoride ion if it completely owned the electron) now believes that it has a full outer shell of eight electrons.
|
Q. How many atoms would you find in the elemental form of Bromine? A. Halogens are diatomic in the elemental form, so there would be two atoms in elemental Bromine (Br2). Each Br atom shares an electron from its outermost shell with the other one, this sharing makes each atom believe that it now has the complete octet, and the covalent bond formed is very strong. |
|
Halogens will form ionic compounds with metals, as opposed to the sharing of an electron, a metal will donate an electron to the halogen atom, forming a positive metal cation and a negative halogen (halide) anion.
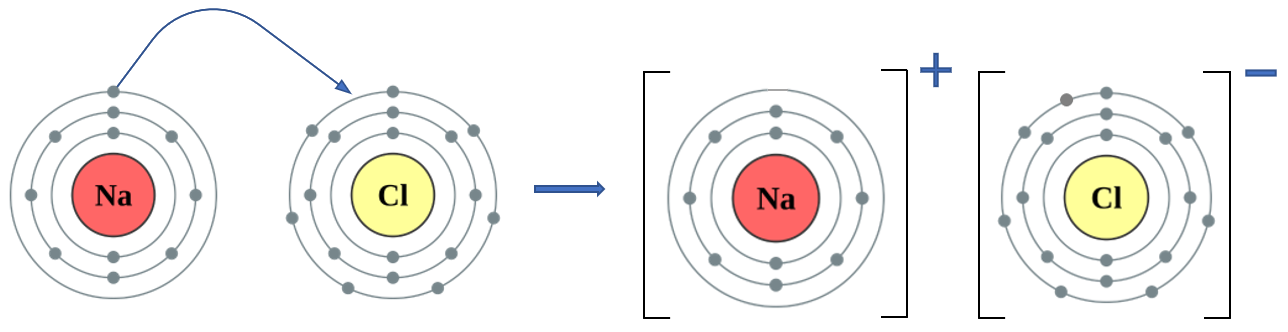
It should be noted here that as the outer shell of the Sodium atom is now empty, drawings could quite correctly omit the shell and show the Sodium cation with only two shells. From a diagrammatical point of view this is OK, but the third shell is still there, it's just got nothing in it. If you count the numbers of electrons in the respective (effective) outer shells now, you should see that Sodium now has a stable octet configuration, as does Chlorine.
However:
This transfer of a single electron from one atom to another has done nothing to the number of protons in their respective nuclei, Sodium still has 11 protons, but now only has 10 electrons. Similarly Chlorine still has 17 protons but now has 18 electrons.
The sodium atom has now become overall positively charged because it has lost one electron, and the chlorine atom has now become overall negatively charged because it has gained one. This means that the newly formed sodium (positively charged) cation and the newly formed chlorine (chloride, negatively charged) anion have opposing charges and will therefore attract each other. It is this strong electrostatic bond which holds these ions together in their lattice.
This is an example of ionic bonding.
We have now seen that group 7 elements will form covalent bonds with each other and with other non metals, and will form ionic bonds with metals. We saw in the group 1 elements, and order every activity in that Lithium was less reactive than Sodium which was less reactive than Potassium and so on down to Francium.
There is also an order of reactivity in the group 7 elements, and the reason for the changing reactivity correlates quite well with the group 1 elements. Fluorine is the most reactive halogen, far more reactive than Chlorine, which is more reactive than Bromine and so on down to (presumably) Tennessine although we don't know much about this particular element at the moment.
Fluorine has only two electronic shells, one of which is missing one electron to make the stable octet. The electrostatic attraction between the Fluorine nucleus and the outer shell is quite high and as a result Fluorine atom would very readily accept an extra electron to make the stable configuration of its next-door neighbour Neon.
Between the nucleus and the outer electrons of a Chlorine atom lies an extra, complete shell of electrons, these tend to "get in the way" of the nuclear-electronic attraction and as such a Chlorine atom is less readily inclined to accept an extra electron, it will of course accept one but not as voraciously as Fluorine will. Therefore Chlorine is overall less reactive than Fluorine.
Proceeding down the periodic table, and for the same reasons, we see that Chlorine is more reactive than Bromine, Bromine is more reactive than Iodine and Iodine is more reactive than Astatine.
The halogens will undergo "displacement" reactions where the more reactive halogen will quite happily "kick out" the least reactive halogen in a compound in aqueous solutions of their salts. These "displacement" reactions are a popular practical exercise in the school laboratory and the results are quite remarkable if performed correctly.
Q. What would happen if Iodine was introduced to an aqueous solution of Sodium Chloride?
A. Nothing. Chlorine is more reactive than Iodine and already exists in solution, therefore the Iodine entering solution would not be able to displace the Chloride ions.
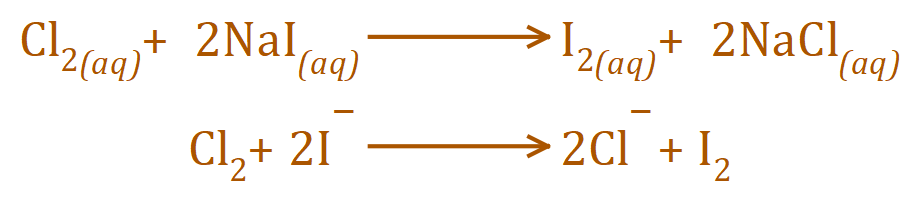
If however a solution of Chlorine water were added to an aqueous solution of Sodium Iodide there would be a displacement of the Iodine anions (Iodide ions) by the Chloride ions to produce a solution of Sodium Chloride with displaced Iodine present.
>> Questions <<
Back To >> [A] Oxidation State <<