[A] Oxidation State
The "Oxidation State" is defined in the image below:
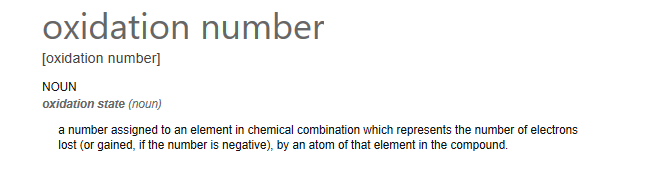
When we talk about chemical reactions involving ions, it is invariably the case that there will be a loss or gain of electrons by one or more parties in the reaction. For example, when we dissolve a small piece of Sodium in cold water, the Sodium atoms are oxidised to Sodium cations by the loss of their single outermost electron:
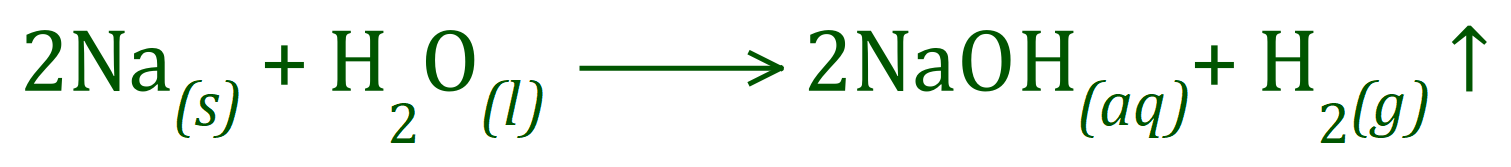
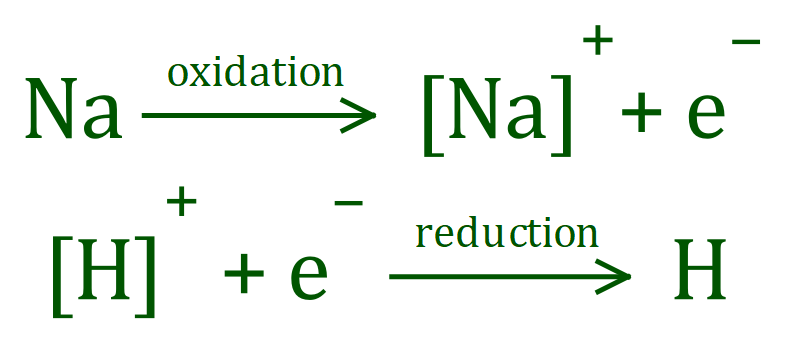
The oxidation state or oxidation number is indicated by the number of electrons lost or gained, and a plus or minus superscript to indicate whether this is a loss or a gain (a + superscript means that there has been a loss of electrons, and a minus superscript indicates that there has been a gain of electrons.
We can recall the acronym "OILRIG" so we know that oxidation is a loss of electrons, which will be indicated as a positive superscript, and reduction is the gain of electrons which will be indicated as a negative superscript.
In the case of the Sodium atom, there has been an oxidation (a loss of one electron) which means that the Sodium ion now exists in a positive oxidation state, it's oxidation state would be indicated as "1+"
In the case of the Hydrogen ion, there is a gain of one electron (a reduction) which changes the "1+" oxidation state of the Hydrogen ion to 0 ("1+" + "1-" = "0"). An element with an oxidation state of zero is uncharged (in other words it's not an ion).
Generally speaking, the oxidation state can give an idea of the group from which the element comes, as most elements have a very limited selection of possibilities when it comes to oxidation state. For example, as we have already seen Sodium (and the rest of the Group 1 Alkali Metals) can either be oxidation state zero, or oxidation state 1+ as an ion.
Calcium, a Group 2 Alkaline Earth Metal, when ionised (as it would be, for example in the compound Calcium Sulphate) exists in an oxidation state of "2+". If we go across to the non metallic side of the periodic table and look at the Group 5, Group 6 and Group 7 elements we can look at some of the negative oxidation state that can exist, for example let's consider the science teachers favourite, Sodium Chloride:
Q. Given the information the equation, state the oxidation number of the ions present.
A.
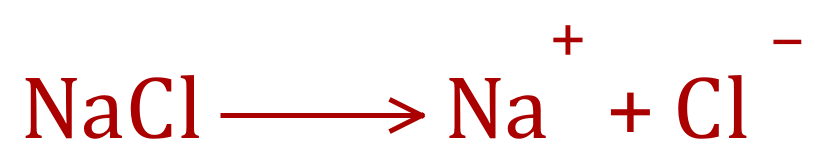
You should be able to see that in this case in the ionic compound sodium chloride, sodium exists in "1+" oxidation state and chlorine (as chloride) exists in "1 -" oxidation state. The same situation exists in Group 6 , Oxygen and Sulphur generally existing with an oxidation number of "2-", however Sulphur deviates from this occasionally in certain compounds.
The elements of group 15 generally exhibit -3, +3 and +5 oxidation states. The tendency to exhibit -3 oxidation state decreases as we move down the group due to an increase in the size of the atom and the metallic character.
Summary:
Generally - Group 1 metals show an oxidation state (or number) of 1+ (or +1), Group 2 metals show a similar tendency to have an oxidation number of 2+ (or +2), and although we have not discussed Group 3, Aluminium for example usually shows oxidation state 3+ as in the oxide Alumina:

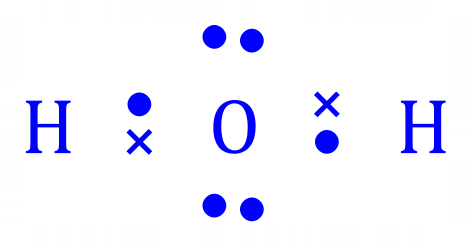
Group 6 non metals such as Oxygen routinely have 6 electrons in their outer shell [2,6] but by covalent bonding can exist in the oxidation state 2- as in the case of Water:
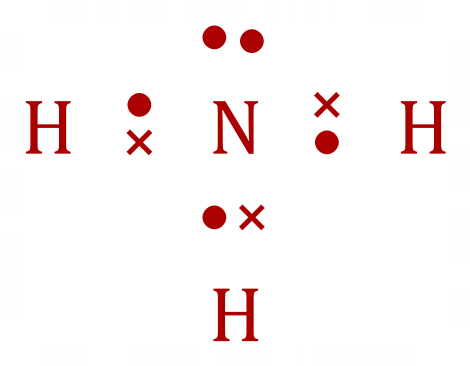
Group 5 elements, such as Nitrogen, exist in a number of oxidation states. In the case of Ammonia below, the oxidation state is 3- as each Hydrogen atom provides (covalently) an electron, thereby entering itself into the 1+ oxidation state, and as we can see by now......"3-" + 3 x "1+" = 0.