Electrolysis
Electrolysis is the breaking down of an ionic compound by passing through it an electrical current. The chemical compound must be in one of two states:
1. Molten
2. Aqueous Solution
In the solid-state the ionic compound cannot conduct electricity as the ions are bound into place it is only when the ions are free to move that they can conduct an electrical current.
|
Electro |
Lysis |
|
A combining form, representing electricity or electric in compound words such as "electromagnetic" or "electrostatic" |
A combining form, representing "breaking down", "loosening" or "decomposition" |
So, what can be electrolysed and what products can we expect? Well, in answer the first part pretty much any ionic substance in aqueous solution or in the molten state. To answer the second part isn't quite so straightforward and depends on the state of the substance being electrolysed, i.e. molten or aqueous solution. If you wish to look at ions to refresh your memory, click >> here << or simply navigate to the next section.
As we said previously electrolysis involves breaking down chemical compounds in the molten or aqueous state by passing electricity through it. What you get, and how you get it is largely dependent on the reactivity or otherwise of the substance or substances concerned.
A little bit more jargon to contend with before we can actually get stuck into electrolysis. You will have heard of the expressions "cathode", "anode", "cation", "anion" and also "oxidation" and "reduction". To understand electrolysis thoroughly you will need to know very clearly the meaning of these expressions, fortunately ingenious minds have come up with ways to help you to do this.

In "general" chemistry, if we talk about a substance being oxidised we are referring to the fact that it has had oxygen added to it, or has had hydrogen removed from it. Similarly if we talk about a substance being reduced it has had hydrogen added to it or oxygen taken away from it.

In this oversimplified example, Iron (when it starts to go rusty) is being oxidised by the addition of Oxygen, to Iron (III) Oxide. When we start looking at "electrochemistry" we have to take on board a slightly modified meaning for the expressions "oxidation" and "reduction".
|
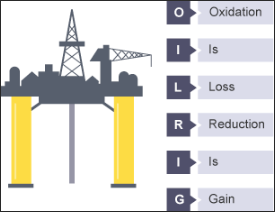
Oxidation |
The loss of one or more electrons |
|
Reduction |
The gain of one or electrons |
If "oxidation is loss" and "reduction is gain" I can feel a mnemonic coming on :-) - O.I.L.R.I.G.
|
|
|
|
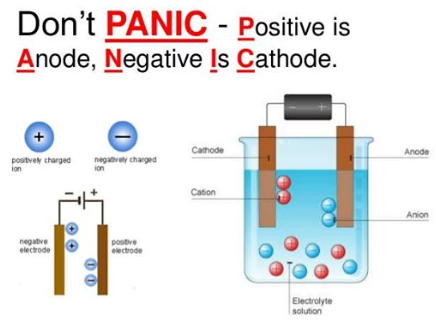
Positive CATIONS migrate towards the Negative CATHODE |
|
Negative ANIONS migrate towards the Positive ANODE |
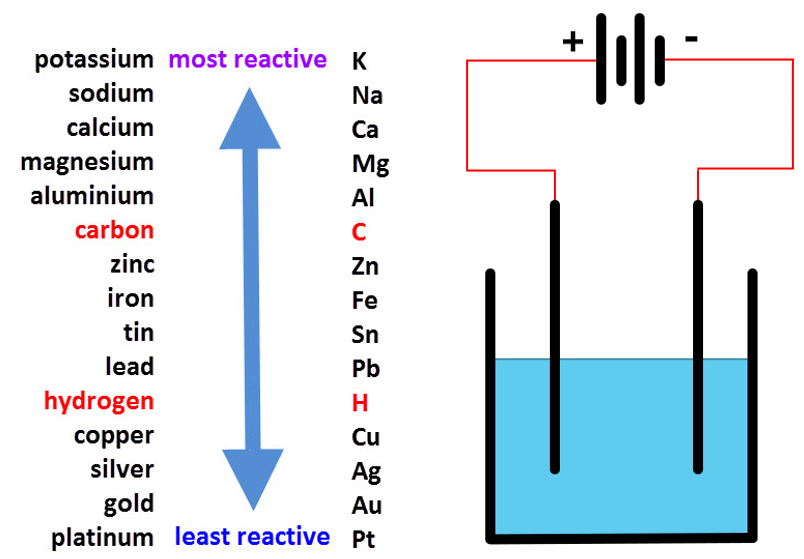
The left-hand side of the diagram shows the reactivity series of metals and the right-hand side of the diagram is a somewhat simplified version of an electrolysis setup. The products of electrolysis depend on where the constituent ions lie in the reactivity table. Hydrogen and Carbon are shown in red because they represent special cases.
Back To >> Tests For Ions <<