Paper Chromatography
There are a number of different types of separation techniques, enabling us to separate mixtures. We have simple filtration, distillation, fractional distillation and this topical willtalk about chromatography. Paper chromatography is essentially a qualitative process, it can tell you what is there (to a certain extent anyway) but not necessarily in what quantities. It is a simple process and is essentially used to separate mixtures made up of liquids of different colours. More serious applications of chromatography can be used in DNA and forensic research. For the purposes of this required practical, we will consider simple paper chromatography.
Q.In paper chromatography why do we draw a line in pencil where the samples have to go?
A. Chromatography relies on the solubility of the substances being separated. A pencil mark is made of graphite and is insoluble, it will therefore not dissolve in the solvent and interfere with the results.
The chromatogram is created by cutting out a square/rectangle of chromatography paper and by drawing a horizontal pencil line approximately 10 mm from the bottom. The solvent is placed in the beaker to a depth of approximately 5 mm. It is important that the solvent in the beaker does not touch the baselineas this will simply serve to wash away the samples. The sample spots are placed at spaced intervals along the baseline and the chromatography paper is placed into the 5 mm (or thereabouts) of solvent. Often water can be used but sometimes depending on what's been tested other solvents such as ethanol may be considered.
Q. Why is it important to make sure that the solvent spots are not immersed in the solvent, or that the solvent does not reach the level of the baseline?
A. This is to make sure that the solvent samples are not drawn into the solvent, otherwise the samples would soak into the solvent and ruin the experiment.
As the solvent soaks its way up the paper, the solvent can be seen marking a visible borderline between wet paper and dry paper. This boundary, which is advancing, is called the solvent front. When the solvent front meets the sample spots the different components making up the samples will advance up the paper at different rates. A lid will be placed on the top of the beaker to make sure that the solvent does not evaporate, particularly in the case of volatile solvents such as ethanol. When the solvent front is almost at the top of the chromatographic paper, the paper will be removed from the beaker and another pencil line is made at the solvent front. The paper is then allowed to dry. The resulting pattern of spots on the paper is known as the chromatogram.
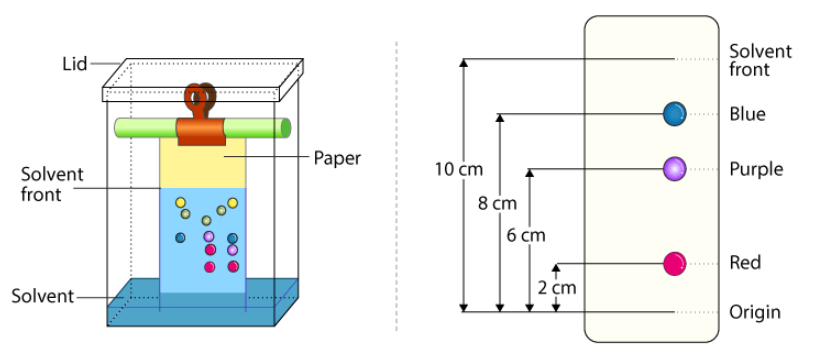
The picture above shows an example of a completed chromatogram, although they are never this clear-cut. This example will serve to demonstrate the arithmetic which follows the chromatography, and that is the calculation of an entity called the "retention factor". The retention factor is a numerical value between zero and one and helps to identify the substances. The retention factor is calculated by dividing the distance from the baseline to the centre of the spot, by the distance from the baseline of the solvent front. This leaves you with a value between zero and one.
For example using the purple spot we can see that the distance travelled by the spot from the baseline is 6 cm the solvent front has actually moved 10 cm. The retention factor value is therefore six divided by ten, or 0.6. The retention factor has no units.