Simple Distillation and Fractional Distillation.
In the previous section we have seen how to separate soluble and insoluble solids such as rock salt and sand. We have also considered that the solubility or otherwise of two salts to determine the way to separate the two if they should get inadvertently mixed up.
During filtration, for example the filtration of sodium chloride solution (rock salt) and the sandy impurities, we have seen that the sand collects in the filter paper but that the dissolved salt and water pass straight through into the flask, ultimately being separated by crystallisation/evaporation. What if we wish to recover the liquid and not the salt?
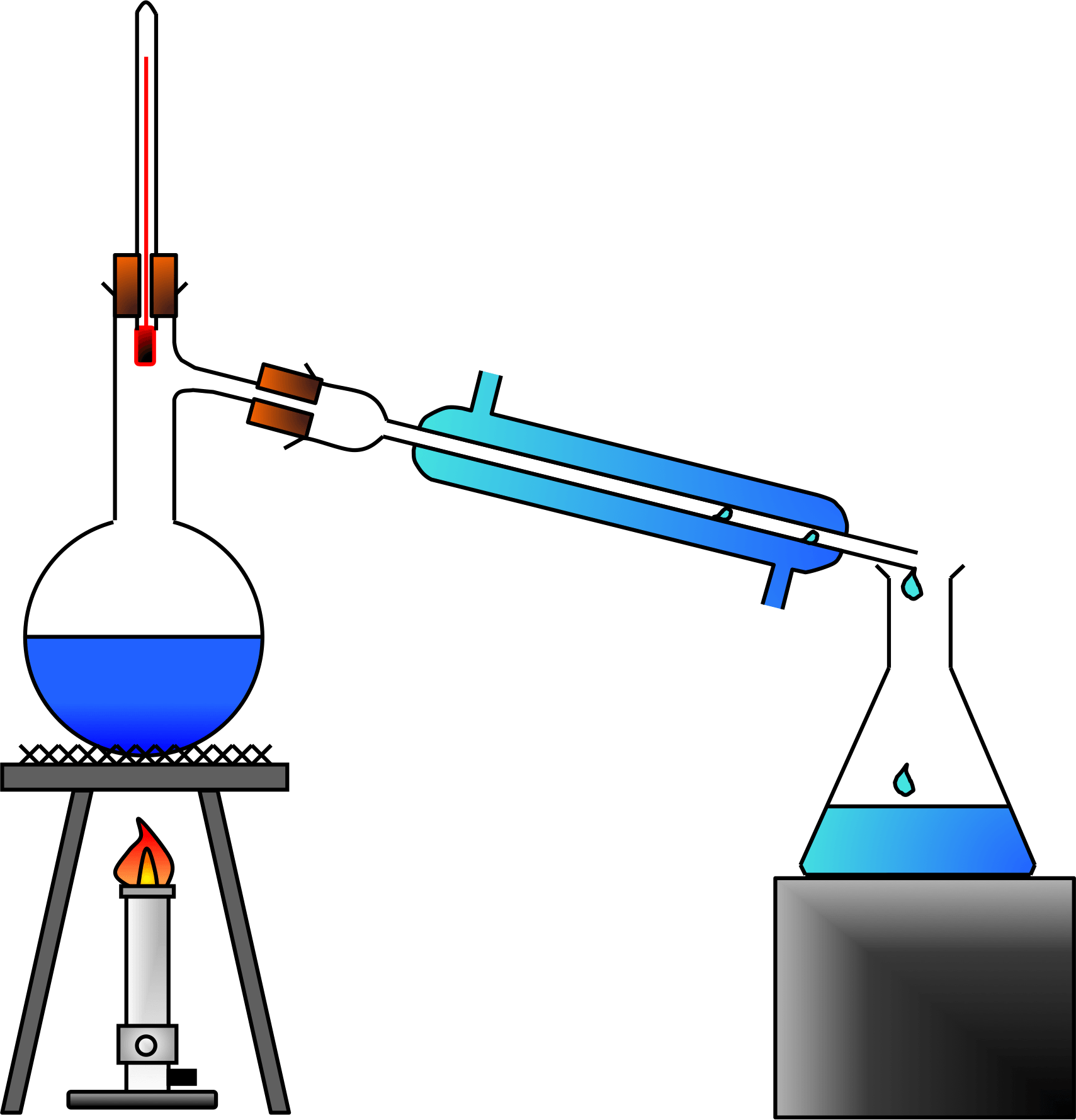
In this case we would need to use a different technique, that of simple distillation. The GCSE examinations contain a required practical in which a student will be given a sample of seawater (or a seawater type of solution) and will need to devise a way, and carries out, of separating the water from the impurities in the seawater, that is to get the water away from the salt dissolved into it.
Industrially pure water is obtained from seawater by this distillation process.
Simple distillation is used when the substances to be separated have widely separated boiling points, or for example if one of the substances is a solid impurities such as the sand (and of course the dissolved sodium chloride) would be in the sample of sea water and sand. We would need to reconsider this particular method if we were looking at substances with fairly close boiling points, because simple distillation would not give you sufficient control over the evaporation process to avoid simply boiling off all of your mixture and re-condensing it, leaving you no further forward.
Q. The student has a mixture of two hypothetical liquid substances X and Y. The boiling points of X and Y respectively are 40°C and 125°C. Suggest a suitable way by which these two substances could be separated.
A. To answer this question we need to make one or two assumptions. Assuming that the objective is simply just to separate them and not worry about losing a little bit of each substance, then we could of course consider a "simple" distillation. If we were to heat our solution to, let's say about 60°C and maintain that temperature for some time we could be quite satisfied in the knowledge that all of the lower boiling point substance would completely come away. If the remaining mixture is simply the second substance (in our case substance Y) then we have completed the job, if not then we would increase the temperature until we reached the boiling point of substance Y, boil it off and collect it in a different collection vessel.
The above question of course is hypothetical and relies on finding two liquids with such a large difference in boiling point. The issues that we would normally encounter might be that we need to separate a mixture of alcohols, for example Methanol, Ethanol and Propanol.
The boiling point of these alcohols is 65°C, 78°C and 97°C respectively. Simple distillation would not really work in this case, because as we approach 65° the Methanol "fraction" would come off but would also bring with it some of the Ethanol fraction. So what exactly is "fractional distillation" and how do we do it?
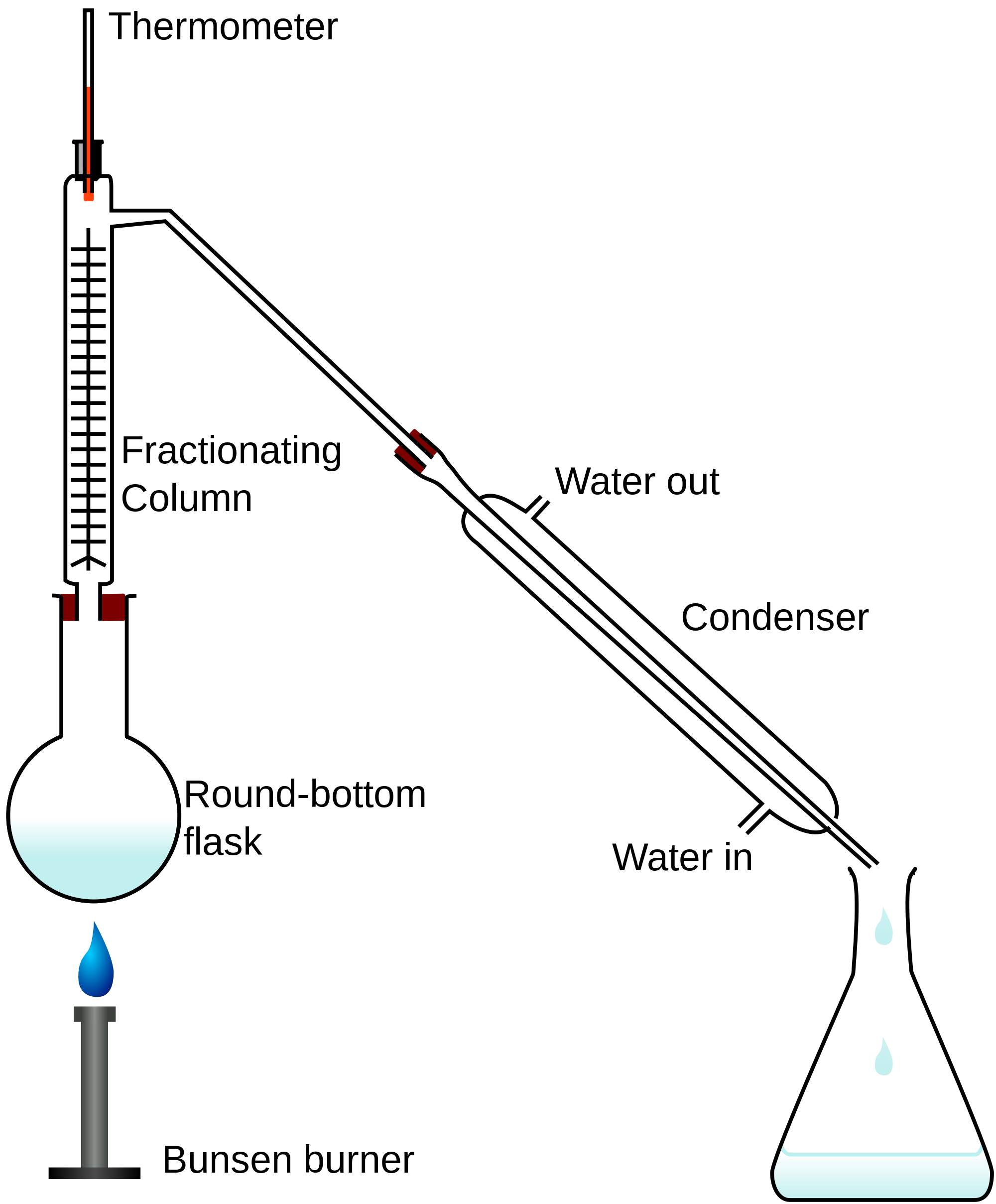
The difference between the diagram to the left and the one above it is that in the second case we use what is called a "fractionating column" but the make-up of this device gives us a little bit more control over the separation of the similar boiling point liquids.
As the mixture is boiled, the "fraction" with the lowest boiling point will reach the top of the fractionating column first (where you would see the red bulb of the thermometer). Of course because the boiling points are close together the other fractions might also start to rise up the column but because of the large surface area inside the column (due to glass beads or other similar substances providing a large surface area for condensation to take place) then these other fractions will condense and fall back into the flask, leaving only the fraction with the boiling points matching the temperature of the thermometer to pass down into the condenser and ultimately into the conical flask.
In the case of our three alcohol mixture, when the temperature on the thermometer reads around about 65°C we should see the lower fraction (Methanol) in its vaporised form appearing at the top of the fractionating column, and making its way down towards the condenser. If we can maintain the temperature at 65°C or thereabouts we would see ultimately the vapour would slow down or possibly stop because all of that particular fraction (Methanol) will have boiled off. We increase the temperature to around 78°C for the second fraction (Ethanol) to boil off and condense into a second conical flask and finally we would do the same with the Propanol at 97°C.