Filtration Evaporation and Crystallisation
|
|
Filtration and crystallisation are techniques which are usually used to separate mixtures, usually mixtures of substances which are soluble for example sodium chloride and water or copper sulphate and water. The techniques are usually conducted in tandem, that is filtration would be used to remove any impurities in solution followed by crystallisation of the filtrate to extract the solid. In a practical investigation, you may be given a sample of rock salt and be asked to separate the sand, salt and water. The way the you would do this quite simply would be to boil a sample of distilled water and dissolve into it the rock salt. This would produce a murky looking sandy coloured solution which is in fact a mixture of salt in solution and sand in suspension (because sand does not dissolve in water). |
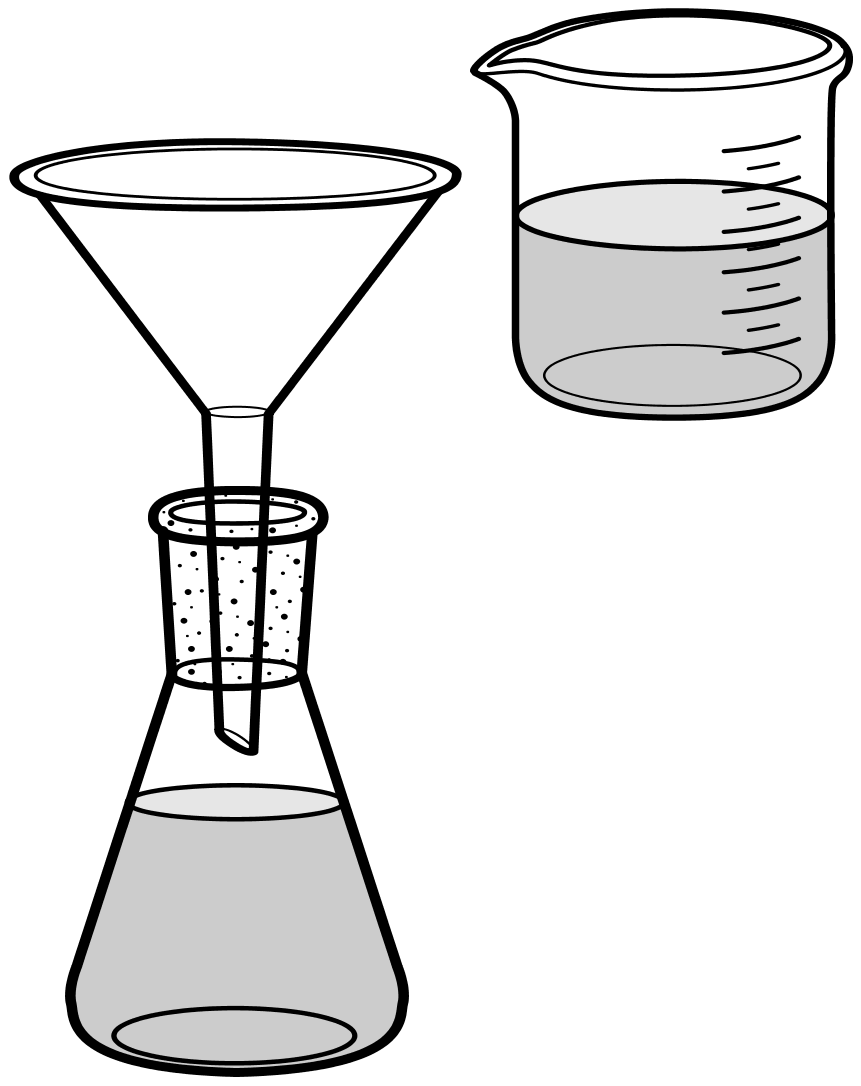
The sandy coloured solution (which would be in the beaker in the diagram to the left) would be passed through the filter as shown into the conical flask and the filtrate collected. Now the filter funnel (which would normally contain the filter paper although one has not been shown here) will catch all of the sand which can be dried if it is required to be kept or discarded and the filtrate passing through into the flask will contain the dissolved NaCl.
The contents of the conical flask would then be passed to an evaporating basin and the solvent (water in this case) would be allowed to evaporate off until the salt crystallised out. Normally to speed this process up an evaporating dish would be used with a heat source to evaporate off more rapidly most of the solvent until crystallisation started, then the process would be allowed to continue naturally until all of the solvent has gone.
This filtration technique is known as "gravity filtration" as it simply relies on the pull of gravity to draw the solvent and the dissolved substances through the filter paper and down into the flask. Another method of filtration uses a device called the Buchner funnel which is usually connected to a vacuum source, the solute is pulled by suction through the filter paper and into the flask.
|
|
In the diagram shown at the top of the page there is a gap between the neck of the filter funnel and the open neck of the conical flask (although the former would normally be held in place using an O-ring or a clamp and stand). Why do you think that this air gap exists? |
Look at the lower picture, the area that I have indicated in light blue is in fact trapped air. Because the filter funnel is close-fitting to the top of the flask it has unintentionally produced a seal which I have shown with a red ring.
As the filtrate is poured into the filter funnel and slowly makes its way through to the flask, the level of filtrate will start to rise in the flask. If the air cannot escape it will push back and it's only way out is back up through the entrance of the funnel because of the "unintended" seal. This will have the effect of pushing back the filtrate and ultimately slowing down to a standstill the filtration process. Many scientists have been known to put a simple piece of filter paper between the neck of the flask and the filter funnel if suitable apparatus has not been available to hold the two apart.
Q. A sample of silver iodide is inadvertently mixed with a small sample of silver nitrate which has been put ready for another experiment. We need to be able to separate these substances. You have been told that silver nitrate is soluble in water but that silver iodide is not soluble in water. Suggest a method by which you might separate the salts.
A.
|
Silver Nitrate |
Silver Iodide |
|
Silver nitrate will dissolve in water. |
Silver iodide will not dissolve in water. |
This additional information should push you towards dissolving your mixed up salt in water until you believe that all of the silver nitrate has been dissolved. The silver iodide will form a suspension because it does not actually dissolve.
While the solution is hot or at least warm, pass the filtrate through a suitable grade filter paper and this will catch the suspended but not dissolved silver iodide. Allow the silver nitrate solution to evaporate and the silver nitrate will crystallise out. This method is suitable to separate these two salts.