Ions
Simply put, an Ion is an atom, or group of atoms which has either lost or gained one or more electrons. By doing this the atom or group of atoms will have gained, or lost, one or more units of negative charge.
This applies to the atom as a whole and to the group as a whole.
If an atom or group of atoms loses one unit negative charge, then that atom or group becomes unit positively charged. Conversely if an atom or group atoms gains one unit negative charge, then it (the whole entity) becomes overall unit negatively charged. This also applies if the atom or group loses more than one or gains more than one electron. A couple of examples will hopefully make this a lot clearer.
One of the favourite elements that we use quite a lot when talking about irons and electrolysis is Sodium, so not to break with tradition, Sodium is the atom that I will discuss here.
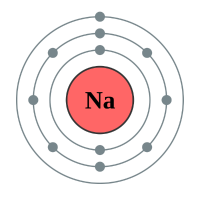
This is the currently accepted portrayal of a Sodium atom. If you count the grey dots you will find that there are 11 of them, and that the grey dots are spread across 3 energy shells. This tells us quite a lot about Sodium, because there are 11 "grey dots" (of course these are electrons) then we know that Sodium in its neutral state will have 11 protons. We can see that the outermost shell contains one electron which puts it in group 1 and that there are 3 energy levels, or shells which makes Sodium a period 3 element.
You probably already know that Sodium is a quite highly reactive metal, reacting quite violently with water to produce Sodium Hydroxide solution and liberate Hydrogen gas. It does this by losing the outermost electron and becoming a Sodium ion.
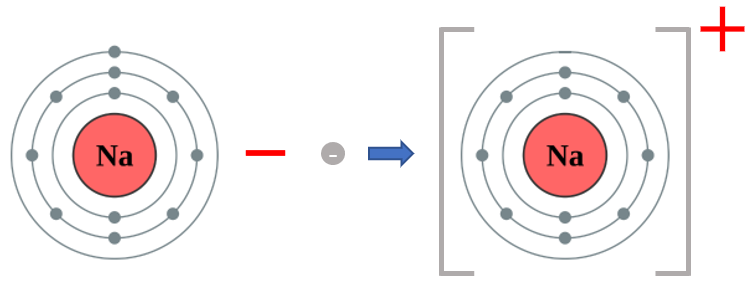
The diagram above explains that a Sodium atom will lose an electron to form a positively charged Sodium ion. If you think about it, 11 electrons orbiting the nucleus containing 11 protons will be overall neutral because 11+ -11 is 0, however if we lose one of those electrons we end up with a mathematical expression of 11+ -10 equals +1, and overall unit positive charge on our newly created Sodium ion.
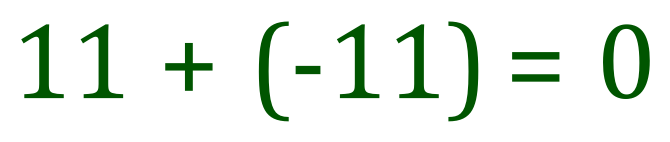
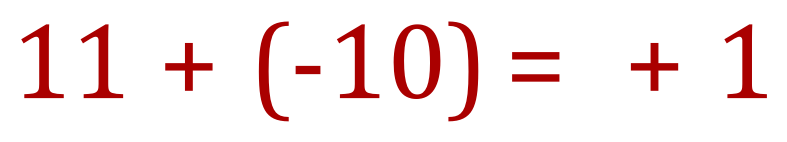
I will go through the process again once using Chlorine this time, because once again Chlorine seems to be a favourite amongst science book authors. Chlorine atoms gain electrons to become chloride ions:
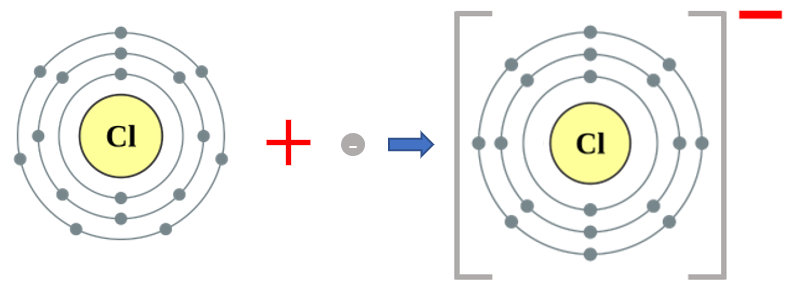
This time a neutral chlorine atom with 17 electrons gains an extra electron (to satisfy the octet rule, see another part of this book) and by doing so imparts a unit negative charge across the whole atom.
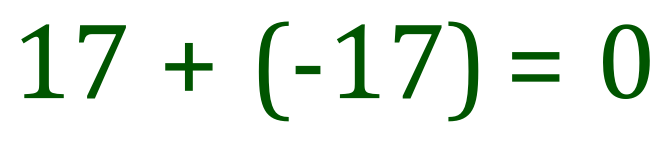

The use of square brackets around the ion is to reinforce the fact that the additional charge (whether it's positive or negative) applies to the whole atom or group, this is perhaps more relevant when we start looking at groups.
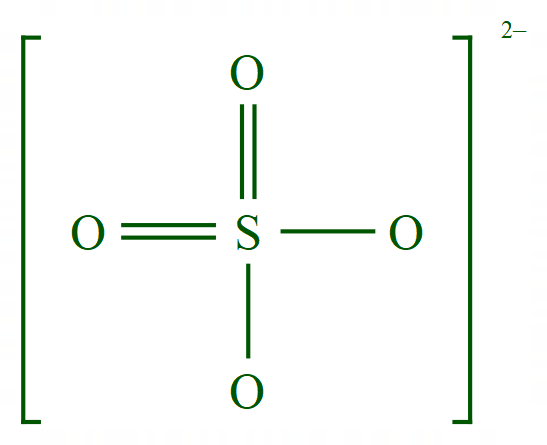
Groups atoms can also form ions, more often than not at GCSE level chemistry you will find groups forming a negatively charged ions (these are usually referred to as "anions") let's take for example a simple salt, Sodium Sulphate:
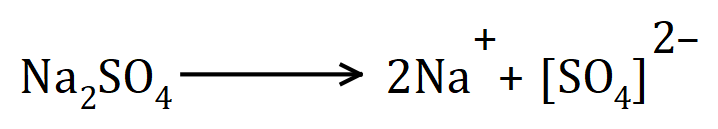
A simple salt derived from the reaction between Sodium and Sulphuric Acid, you can see that two Sodium atoms have each lost an electron becoming individually singly positively charged in the process. (In the reaction these electrons would have been donated to the protons making up Sulphuric Acid, these would then be released as individual Hydrogen atoms and would quickly pair up to form a Hydrogen molecule which you would see bubbling out the solution).
The original electrons from the Hydrogen atoms in the Sulphuric Acid molecule have been retained by the "Sulphate" group which shows an overall double negative charge. You should be able to understand now that the square brackets represent the fact that this double negative charge is spread across the complete 5 atom unit that makes up the group. When we go a little bit further into this you will also understand why Sodium is more willing to enter solution by losing electrons and become ions than Hydrogen, simply because of the fact that Sodium is higher in the reactivity series which you will come to very soon.
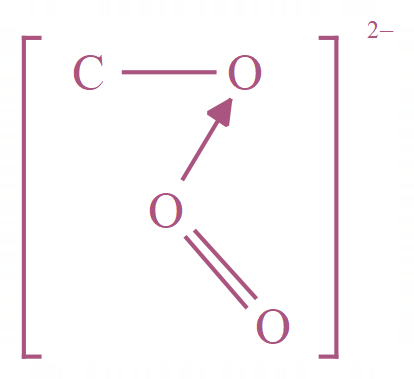
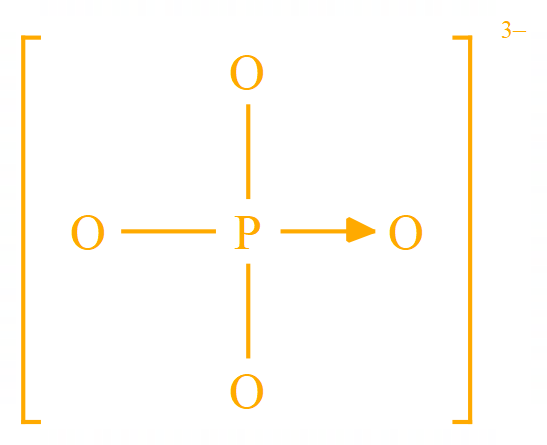
A few more examples of multi atom groups are shown below, hopefully by now you have a good understanding of ions so we can continue with the topic of electrolysis.
|
|
|
|
Nitrate |
Sulphate |
|
|
|
|
Carbonate |
Phosphate |
Now that we have a little better understanding of ions and ionic groups we can return to electrolysis.
Go To >> Ionic Bonding <<