Ionic Bonding
Previously, in the section on "ions" we saw how atoms and molecules can lose or gain electrons to become positively or negatively charged ions. There are a number of factors to consider which will determine how readily electrons can be lost or gained. This section talks about what happens to atoms and molecules once they have become "ionised" and how this "ionisation" leads to ionic bonding.
Metals generally lose electrons to form positively charged cations, non metals generally gain electrons to form negatively charged anions. The reason that this happens is that the atom (talking about atoms exclusively now, not groups of atoms which form positively or negatively charged compound ions) is trying to reach the stable "octet" or "stable eight" configuration. This will give them the same sort of electronic structure as the noble/inert gases and atoms with full outer shells become very stable.
The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.
We will come to covalency later, but suffice it to say that the above definition is sufficient for our needs at this level. Let's take a look at a chemistry favourite, Sodium.
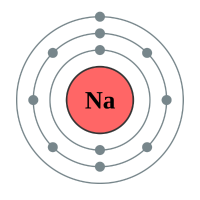
As you probably know by now, Sodium is a highly reactive metal and much of this reactivity is down to the fact that Sodium is quite happy to release its single outermost electron, to drop down to the inert gas configuration of its neighbour Neon.
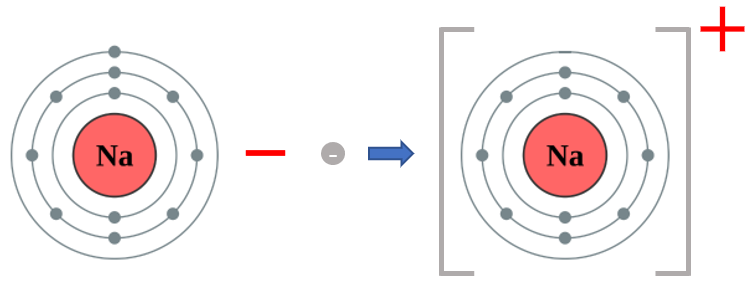
Just as a reminder, if an atom of Sodium loses its electron, it does not become an atom of Neon, because the number of protons in the nucleus does not change, however there becomes a charge imbalance as the eleven protons in the nucleus of a sodium atom now overpowers the remaining ten electrons, leading to a net overall charge for the atom (which is of course now an ion) of "plus one". The oxidation state of Sodium in this particular configuration is +1.
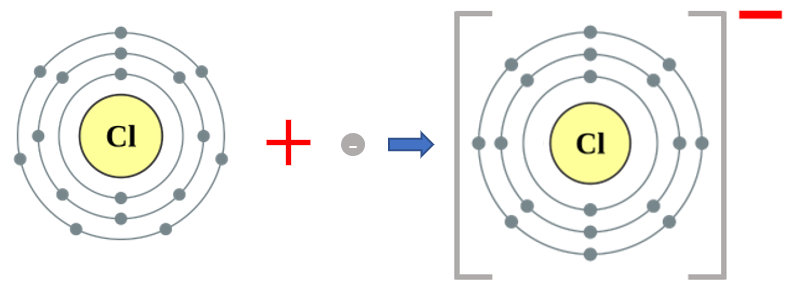
Similarly, if we pick on the element Chlorine we can see that Chlorine has a vacancy in its outer shell, having 7 electrons when it would much rather have 8. As a result of this Chlorine quite readily accepts an electron to form the negative charge Chloride ion. In this incarnation Chlorine (Chloride) exhibits an oxidation state of -1.
Opposite charges attract each other, and the newly positively charged Sodium cation will be attracted towards the newly negatively charged Chlorine (Chloride) anion forming a strong electrostatic (ionic) bond between the two.
In terms of electron transfer:
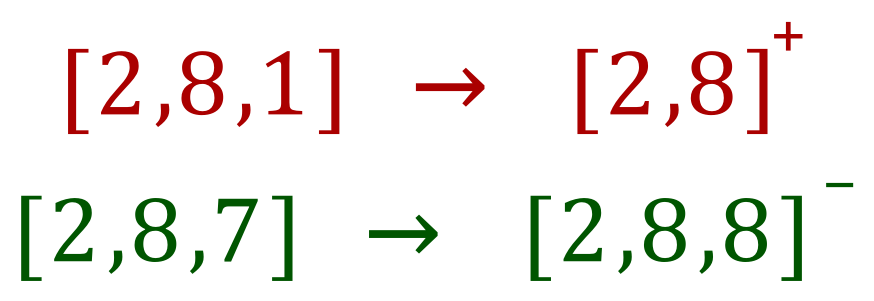
Go To >> Solubility of Ionic Compounds << Chart