Covalent Bonding
Covalent bonding is about "sharing" of electrons so that the participants feel as if they have individually the "stable eight" configuration. Covalent bonding is found between nonmetals such as in Hydrogen, Oxygen the Halogens and even compounds such as Methane and Water.
The best way to show this "sharing" is by using the "dot and cross" representations that you have met before, for example let's take a look at the simplest molecule Hydrogen.

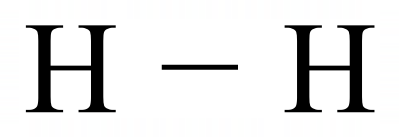
A Hydrogen atom has only one electron, the innermost shell of an atom can hold only two electrons and so if a Hydrogen atom could obtain a second one from somewhere it would have the stable electron configuration of the first inert gas, Helium. A Hydrogen atom looks to another Hydrogen atom, now if they were to "share" their single electron then that would make them both feel as if they have two electrons. They could happily coexist join together in this way forming a diatomic Hydrogen molecule.
This "sharing" or covalency extends beyond hydrogen of course, to other nonmetals and nonmetallic compounds but the situation does start to get more complicated.

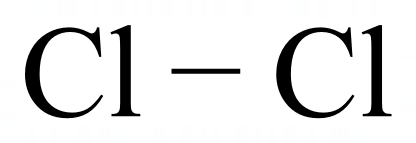
The full electronic configuration of the Chlorine atom is [2, 8, 7] which tells us that a Chlorine atom has three occupied electron shells, the first two of which are full and the third being deficient by only one electron.
Chlorine is very close to the stable electronic configuration of Argon and needs to get hold of that extra electron somehow to achieve it. Two Chlorine atoms get together and share their "unpaired spare" electron in a covalent bond, which makes each chlorine think that it has eight electrons and has achieved its "electronic Nirvana" of the stable gas configuration of Argon.
This provides us with a single covalent bond between two Chlorine atoms, forming a very stable diatomic Chlorine molecule. However, this is not the end of the story because some molecules have double or even triple bonds between participating atoms. We will take a look at the two commonest ones now, being Oxygen and Nitrogen.
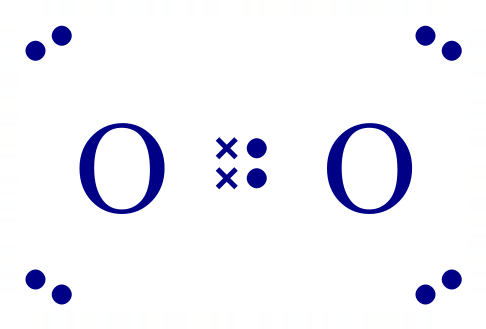
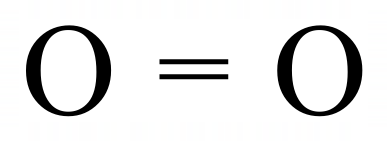
The electronic configuration of a single Oxygen atom is [2, 6] which tells us that the innermost shell is full, but that the outermost shell is missing two electrons to take it to the "stable eight" configuration. In much the same way as chlorine, Oxygen finds the remaining necessary electrons by sharing two of its existing electrons with another Oxygen atom. This forms a double covalent bond where each atom contributing to the bond believes that it has full ownership of the four electrons making the bond, and therefore holds the stable eight configuration. Remember that it takes two electrons to make a single bond so four electrons will make a double bond. You probably won't be surprised to learn that Nitrogen produces a six electron triple bond between two atoms to form its diatomic natural state:

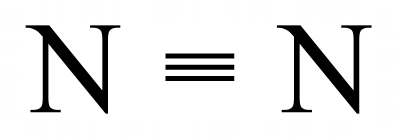
The electronic configuration of a single nitrogen atom is [2, 5] so it needs to fire three more electrons to achieve the stable eight configuration. As you can see it does this by sharing three electrons from another nitrogen atom which produces a six electron triple bond, both nitrogen atoms believing that it has exclusivity over all eight electrons (its own two remaining electrons and the six shared in the bond).
You have the "dot and cross" and the "displayed" structures here, but more often than not you may be asked to show the structure of a covalent bond using overlapping circles just as you would in mathematics in a Venn diagram. It is helpful, but not essential to show the "shared" electrons in the middle of the overlapping segment. Provided that you make it clear that the electrons there are being shared you should be okay.
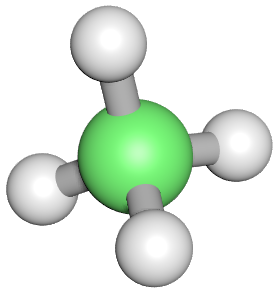
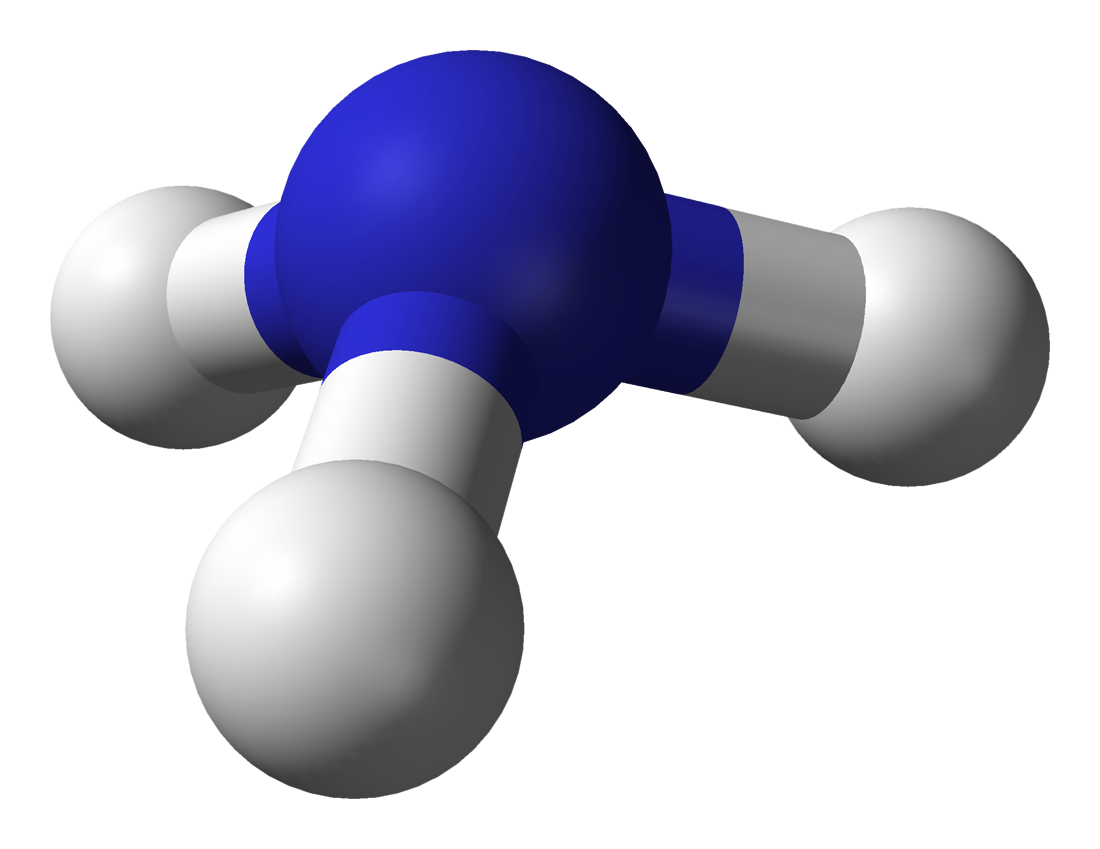
We can use these diagrams to adequately show the electronic make up of the molecule, but this model falls down when we need to know a little bit about the stereochemistry. For example we can show an ammonia molecule, NH3 quite adequately using either of the formats we have already seen, but this doesn't help us to visualise the three-dimensional nature of ammonia and its almost tetrahedral shape. This is where "ball and stick" and 3-D models become useful.
|
|
|
In the case of ammonia, the top cross representation show the "lone pair" of electrons which nitrogen possesses, and not used in the bonding. The lone pair does however contribute to the three-dimensional shape of the ammonia molecule.
A final, simple molecule which is covalently bonded is the simple alkane Methane: