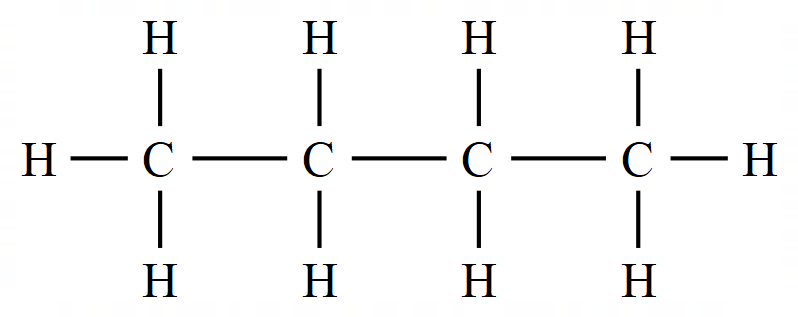
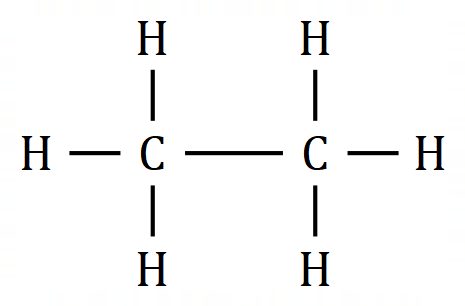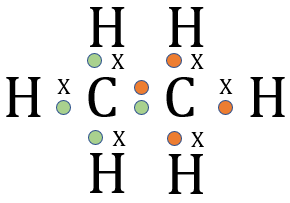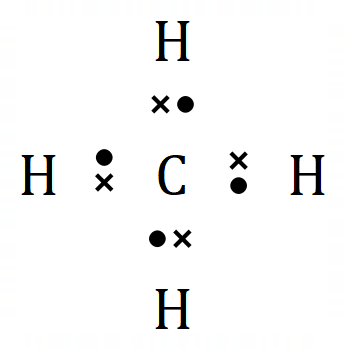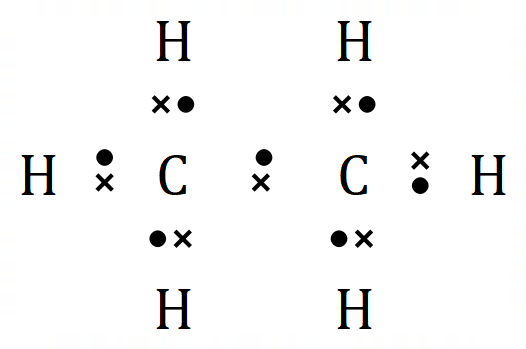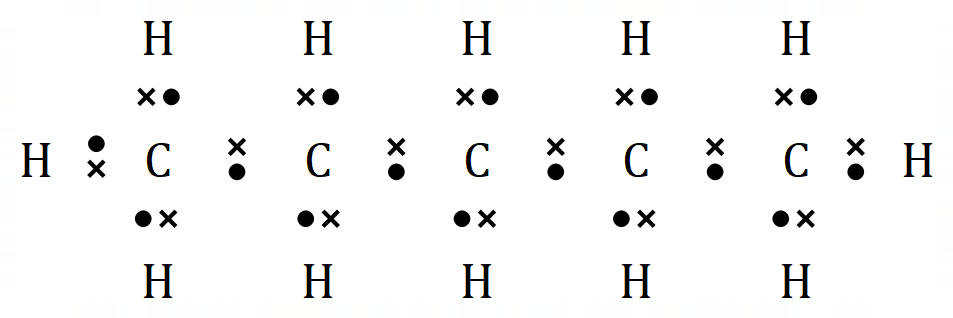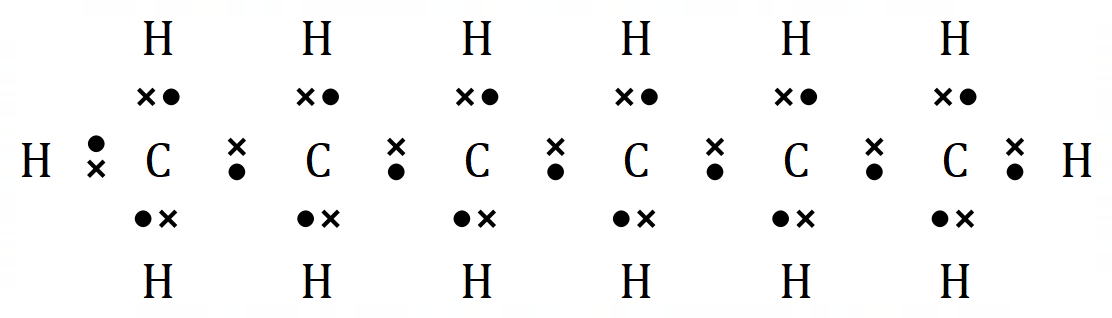Alkanes
Perhaps the simplest substances in organic chemistry of the straight-chain saturated hydrocarbons known as alkanes. The expressions "straight-chain" and "saturated" will become clear shortly (in the latter case, it has nothing to do with the molecules being wet!)
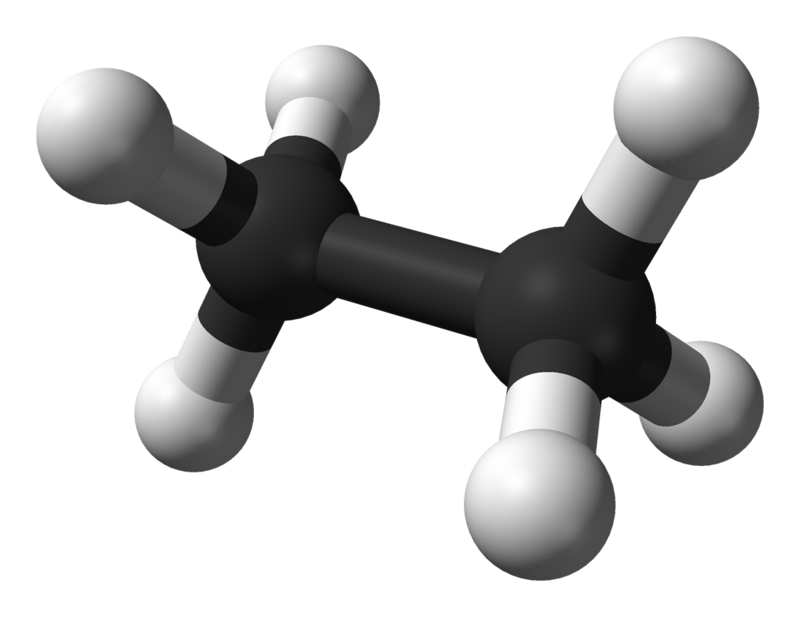
Let's take a look at our first alkane: Methane:
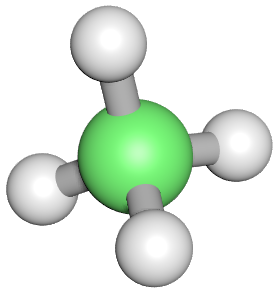
The green sphere in the middle represents one atom of Carbon, the 4 white spheres each represent one Hydrogen atom. The "tube" appearing to join each to the other is a covalent bond comprised of 2 electrons each. It is easier to see this bonding by drawing our molecule in a different way using what is known as a "dot structure".
We know from our studies of inorganic chemistry that it is the "valence" electrons or the "outer shell" electrons that take part in the chemical bonding process, so for example if we consider the element bromine which has 35 electrons in total, we are only really interested in the 7 electrons that lie in the outer shell, and as such when we draw these structures we usually only draw the outer shell electrons.
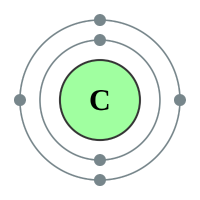
Let us consider the "organic chemistry star of the show" the element carbon:
|
|
|
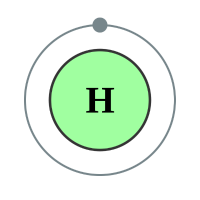
I have also shown the element Hydrogen with its single electron for reasons which will become apparent very shortly. Looking at the element Carbon, we can see that the outer shell contains 4 electrons, these are the "valence" or "valency" electrons and are the electrons which will get involved in the bonding process. Similarly, Hydrogen has one electron in its outer shell (in fact it only has one electron) but is ready to share it with Carbon in a covalent bond during the formation of the compound Methane above.
You can see that the compound methane would have a formula CH4 which means that 4 Hydrogen atoms bond to 1 Carbon atom. Let us use the dot notation above to show how this is done.
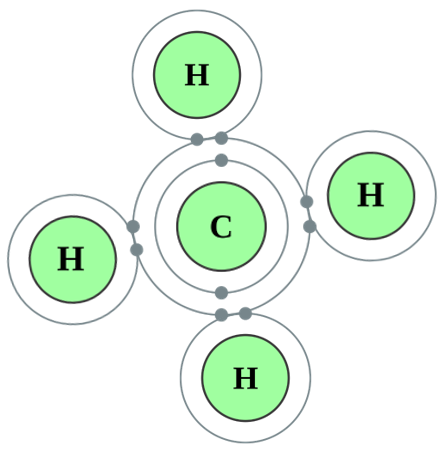
Carbon now has access to 4 more electrons, it experiences the delights of having a full outer shell or "octet" which is the same as its near neighbour, the noble gas Neon. The "stable 8" configuration makes carbon very stable in this arrangement. Likewise, the Hydrogen atoms now have a "full" outer shell of 2 electrons (remember the innermost shell can hold only 2 electrons) and are also very happy and stable this way. This "sharing" as we know it, or "covalent" bonding is a huge feature in the study of organic chemistry.
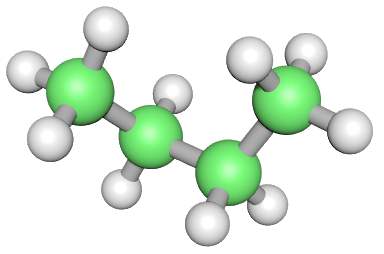
The dot structure is not ideal for showing organic molecules much larger than Methane, we consider a number of other representations such as the 3D "ball and stick" as above when we saw Methane for the first time, or a simpler version of the dot representation.
When the molecules get very large we use an unusual method that takes some getting used to, by not showing H atoms and only representing Carbon atoms by the "stick" that makes the bond:
|
|
|
|
|
3D Spatial Representation |
Wedge and Dash |
Displayed Formula |
|
CH3CH3 or C2H6 |
− |
|
|
Molecular |
Skeletal |
Dot and Cross Notation |
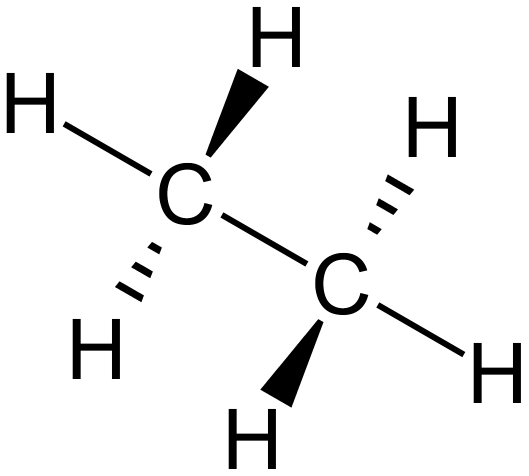
All of the above are valid representations of Ethane. The lower middle (skeletal) one might not look much, but as the molecules grow, it will become a useful alternative indeed.
All of these representations have advantages over each other, the three-dimensional spatial representation gives you a good idea of what the molecule might look like if you were down there small enough to see it. The wedge and dash option allows you to simulate a three-dimensional molecule on a two-dimensional page and the displayed formula is the way that you will see these molecules represented in most books.
The molecular formula is probably the version that you will see when writing out/balancing organic chemical equations and as I've said before, the skeletal version will come into its own when you're dealing with large structured molecules.
The only one I haven't discussed so far is the dot and cross notation. This is a very good way to show the electrons "apparently belonging" to each atom taking part in a bond. Just for the sake of simplicity I have shown the hydrogen atom electrons as X, the leftmost carbon atom electrons are light green circles and the rightmost carbon atom electrons are orange circles.
Of course electrons aren't coloured!
This representation however, does give you the opportunity to see that the carbon atoms and the hydrogen atoms in this arrangement are enjoying the benefits of full outer shells by sharing, this being the whole ethos of covalent bonding.
Let's take a look now that the first 6 alkane molecules, Methane through to Hexane.
For this I will stick to the simple dot and cross formula.
|
|
|
Methane |
|
|
|
Ethane |
|
|
|
Propane |
|
|
|
Butane |
|
|
|
Pentane |
|
|
|
Hexane |
It is reasonably easy to see with just 6 molecules that there is a formula for the general structure of alkanes. For every 'n' Carbon atoms, there will be 'n+2' Hydrogen atoms:
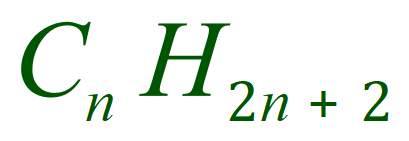
The simplest alkane, Methane is a gas at RTP, as are Ethane, Propane and Butane. When we reach Pentane we start to move into the alkanes which are liquid at RTP and as we move further up the chain, we start to encounter the waxy solids of the longer chain alkanes.
You will in fact hear the Alkanes and the related Alkenes and Alkynes referred to as "homologous series", which is just a very scientific name for a family of such substances. As well as the "generic" formula for Alkanes, there is also one applicable to Alkenes and a third one applicable to Alkynes. The last homologous series, the Alkynes does not appear in the GCSE syllabus.
The reason that the boiling point increase is that as the molecular chain (or as you will see referred to, "backbone") gets longer, there is more scope for entanglement between molecules and attractive forces (such as the Van der Waal forces we have seen before) and so the substances tend to cling together and liquefy, then become viscous liquids before they finally become solids.
|
Name |
Formula |
Molar |
Melting |
Boiling |
Number of Structural Isomers |
|
Methane |
CH4 |
16 |
–183 |
–162 |
1 |
|
Ethane |
C2H6 |
30 |
–183 |
–89 |
1 |
|
Propane |
C3H8 |
44 |
–187 |
–42 |
1 |
|
Butane |
C4H10 |
58 |
–138 |
0 |
2 |
|
Pentane |
C5H12 |
72 |
–130 |
36 |
3 |
|
Hexane |
C6H14 |
86 |
–95 |
68 |
5 |
|
Heptane |
C7H16 |
100 |
–91 |
98 |
9 |
|
Octane |
C8H18 |
114 |
–57 |
126 |
18 |
|
Nonane |
C9H20 |
128 |
–54 |
151 |
35 |
|
Decane |
C10H22 |
142 |
–30 |
174 |
75 |
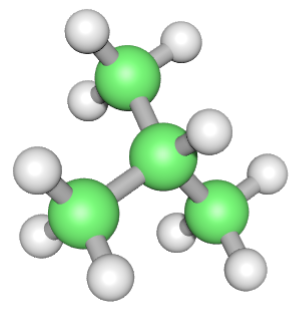
The table makes reference to structural isomers, and these are different ways in which the atoms making up the hydrocarbon can be connected together. Isomerism in the alkanes starts at butane, with C4H10 existing as 2 different structural isomers:
|
|
|
|
|
|
|
C4H10 - Butane |
C4H10 - Isobutane (2-methylpropane) |
Don't concern yourself too much at the moment with the naming of the substances. Isobutane is quite an old-fashioned name, the isomer is correctly named 2-methylpropane because there is a methyl group on the second carbon atom (we will cover naming of organic hydrocarbon and derivative compounds later).
Hydrocarbons burn well when there is plenty of oxygen, to produce Carbon Dioxide and Water. When these are the only two products, the combustion is said to be "complete". There are times when the combustion is said to be "incomplete" and this can be quite dangerous.
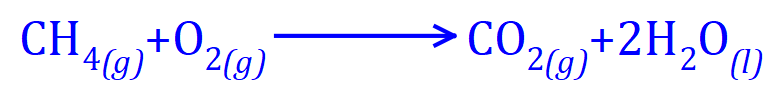
The equation above shows that Methane, completely combusted in Oxygen produces Carbon Dioxide and Water.

Similarly, the complete combustion of Ethane in Oxygen produces Carbon Dioxide and Water, but as we increase in hydrocarbon chain length, we produce more of both products.
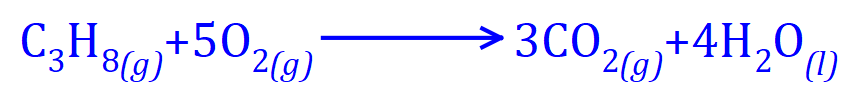
Propane requires a plentiful supply of Oxygen to produce Carbon Dioxide and Water. Study the two previous equations carefully, in the case of Ethane we require 3 1/2 molecules of Oxygen although to save having a fraction in the equation, we doubled up.