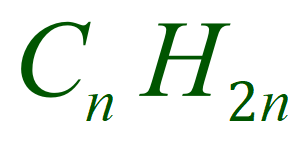Alkenes
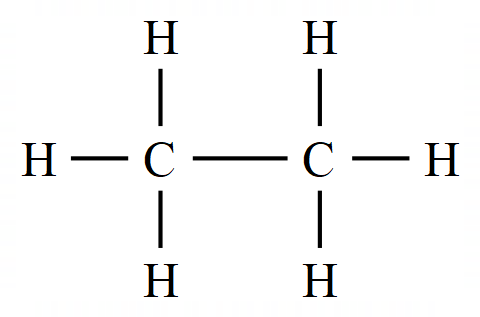
In the previous section on alkanes we talked about (albeit very briefly) "saturated" hydrocarbons and the comment was made about this not meaning that they are soaked in water :-). A saturated hydrocarbon is one where every bond belonging to every carbon atom is in use, in other words carbon and hydrogen are "single bonded":
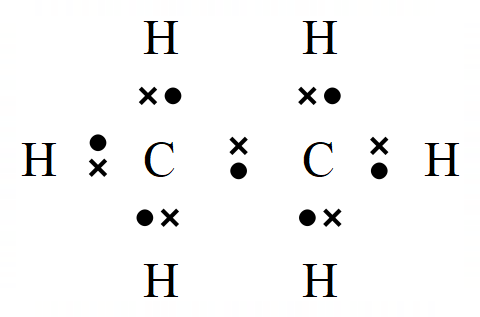
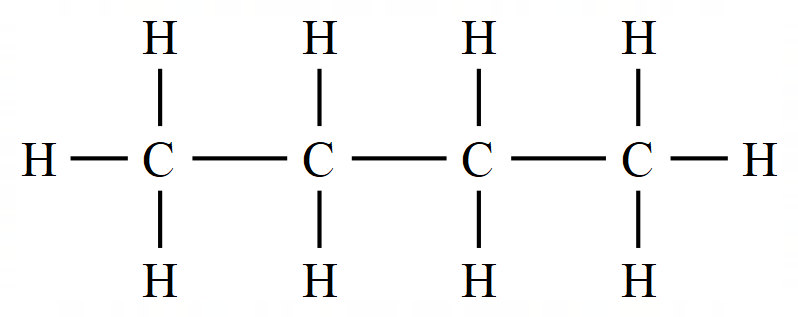
The above diagram represents a molecule of the alkane Ethane. All of the bonding electrons in the carbon atoms and the hydrogen atoms are being used in the formation of chemical bonds, Ethane is referred to therefore as a "saturated hydrocarbon" as all of the bonds are single bonds.
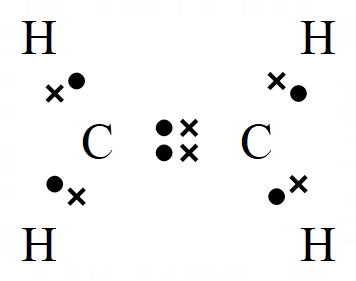
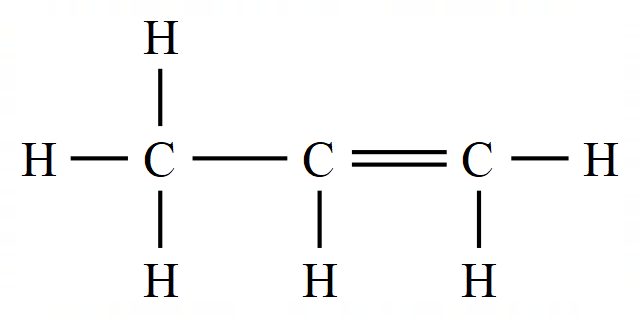
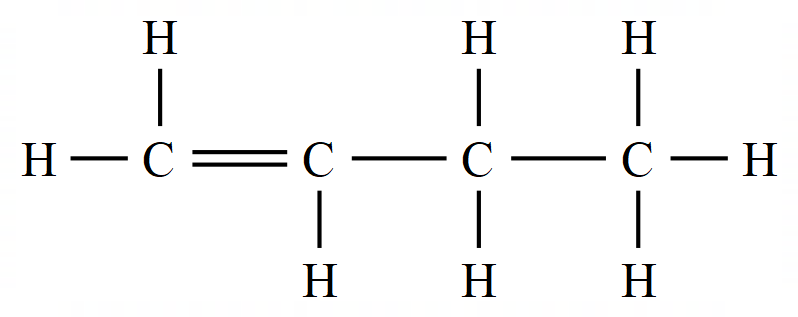
In the Alkenes we have a situation where not all of the bonding electrons provided by the carbon atom are being used to make covalent bonds between hydrogen (or any other substituent atom). In these cases the "unused" electrons form a double electronic bond between the carbon atoms, and this is usually represented as follows:
|
|
|
The representation of Ethene (which used to be called Ethylene) on the left is the familiar "Dot and Cross" representation, however in your studies of organic chemistry you're more likely to come across the right hand representation which has a simpler way of showing the double bond between the carbon atoms.
Alkenes are usually produced when long-chain hydrocarbons are "cracked", that is broken down by thermal decomposition into shorter chain hydrocarbons. The reason that we "crack" long-chain hydrocarbons is that they are not really much use to us in that state, but shorter chain hydrocarbons make good fuels and are in very high demand (for example petrol). As well as producing mid size hydrocarbon chains such as Hexane Heptane Octane and so on, small chain Alkenes such as Ethene and Propene (which used to be called Ethylene and Propylene) are produced and these find a lot of uses in the plastics industry.
One of the ways in which, for example, we can produce Ethene is to "crack" the very long hydrocarbon Decane into Octane and Ethene:

cracking to:

The Alkenes are more reactive than the Alkanes, they have at least one double bond between carbon atoms which is covalent. Because this bond is a double bond and the electrons forming it not being used to bond hydrogen atoms, Alkenes are said to be "unsaturated".
The double bonds can be "opened up" in certain chemical reactions to produce "substituted Alkanes", indeed one of the tests for Alkenes involves opening up the double bond (s) using bromine water, forming a dibromoalkane.The naming or nomenclature of the Alkenes follows a similar pattern to that of the Alkanes, here are the first 3 Alkenes compared to the first 3 Alkanes ( where n >1 ):
|
|
|
|
Ethane |
Ethene (ethylene) |
|
|
|
|
Propane |
Propene (propylene) |
|
|
|
|
Butane |
Butene ( butylene) |
There are two points to note here, first of all I have started at Ethane and Ethene (indicated by the fact that I put 'where n > 1') because of course the cannot be a substance known as Methene (methylene) as a carbon atom cannot have a double bond with itself!
So we start with Ethane, then we move on to Propane and Butane in the Alkanes, similarly we start off with Ethene, followed by Propene and Butene.
Study the examples of the Alkenes and see if you can spot a question, a hint would be to pay particular attention to the largest example, Butene.
Okay, I'll give you a clue, look where the double bond is. Could the double bond go anywhere else? Well yes of course. It could go between carbon atoms 1 and 2 (starting from the left) or carbon atoms 3 and 4, but in both cases it would still be the same substance however there is a difference between Butene with the double bond between carbon atoms 1 and 2, and Butene with a double bond between carbon atoms 2 and 3, and this gives rise to a difference in the way in which the substances are named.
We will look in a little bit more detail later on at the naming conventions for organic substances but for now, just to satisfy the little problem I have raised I will show you the 2 structural isomers and their respective names.
|
|
|
|
1-Butene (or But-1-ene) |
2-Butene (or But-2-ene) |
The carbon atoms are numbered in such a way as to give the double bond the lowest number, so for example in the first case, as a double bond lies between carbon atoms 1 and 2 it becomes 1-Butene. I'm sure you can see therefore that if the carbon-carbon double bond lies between carbon atoms 2 and 3 we correctly name this 2-Butene.
There cannot be a 3-Butene as this would in fact be 1-Butene "flipped" the other way!
Alkenes have the generic formula: