Reactivity of Alkenes
Alkenes are quite reactive substances and find uses as the starting points in the manufacture of other organic substances, for example plastics and so forth. In fact it is useful to remember the "old" names of some of the Alkenes, such as Ethylene and Propylene because when these substances are polymerised (more on that later) they produce substances that you are probably familiar with, such as Polythene (Polyethylene) and Polypropylene, both of which are hard wearing highly useful plastics. (Polypropylene in fact finds good use in doormats, so it must be hard wearing).
We said earlier that if we "crack" long-chain hydrocarbons, usually Alkanes, we can produce shorter chain hydrocarbons including Alkenes. These gases are invisible so how do we know we've got Alkenes? Well, there are tests that we can perform to show the presence of an alkene, and perhaps the most widely known one is the discolouration of bromine water.
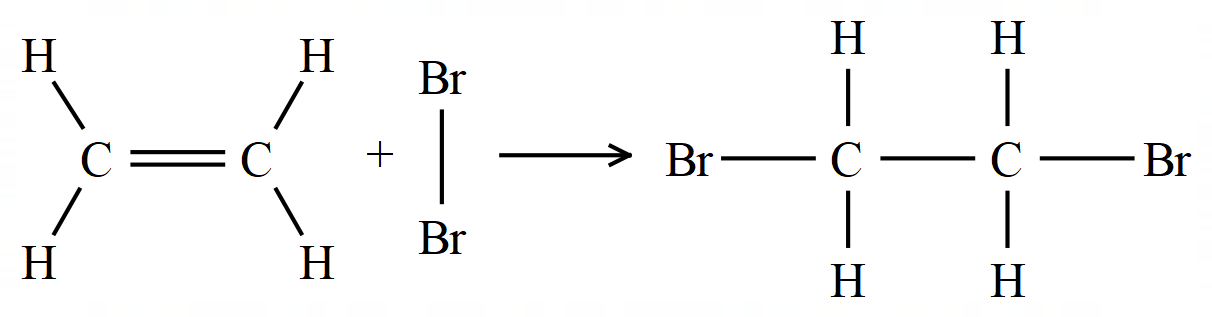
Bromine water, as its name might suggest, is a weak solution of the element bromine in an organic solution. When an alkene is mixed with bromine water, the orange coloration of the bromine water is taken away:
"Nice to know, not need to know"
The above reaction is an example of an electrophilic addition reaction across the double bond between the carbon atoms, the product as you can see is 1,2-dibromoethane. The way in which this reaction takes place is not fully understood and there are currently 2 accepted "explanations" of how this reaction takes place. This part is quite advanced and is not necessary for GCSE, but if you are studying, or planning to study advanced level chemistry, you should start to look at these things.
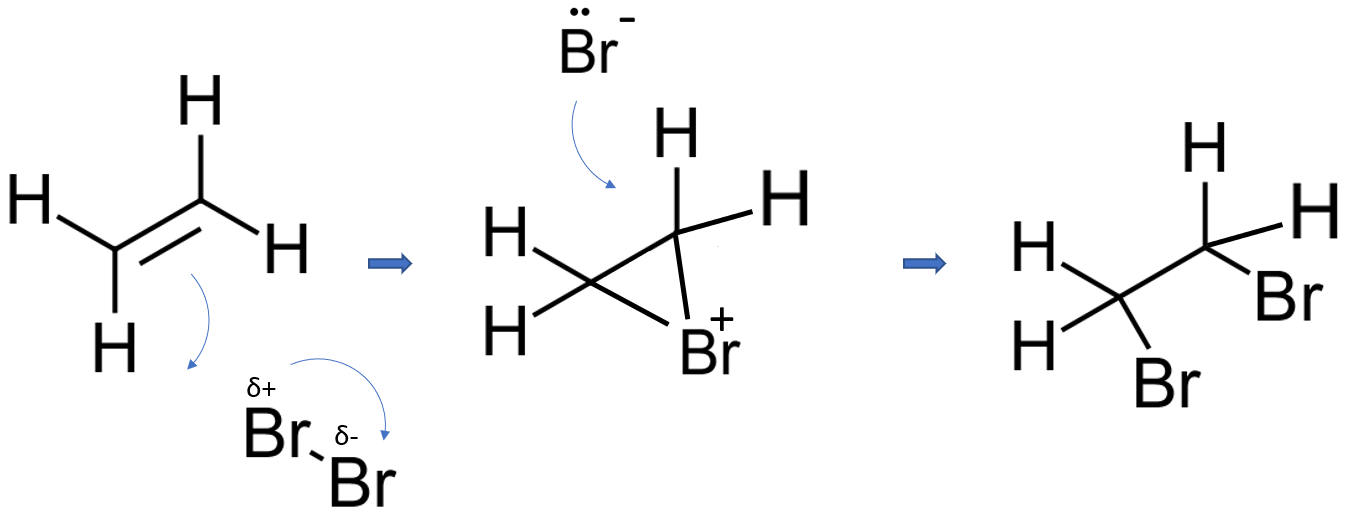
Suggested mechanism 1
The bromine is a very "polarisable" molecule and the approaching pi bond in the ethene induces a dipole in the bromine molecule. If you draw this mechanism in an exam, write the words "induced dipole" next to the bromine molecule - to show that you understand what's going on.
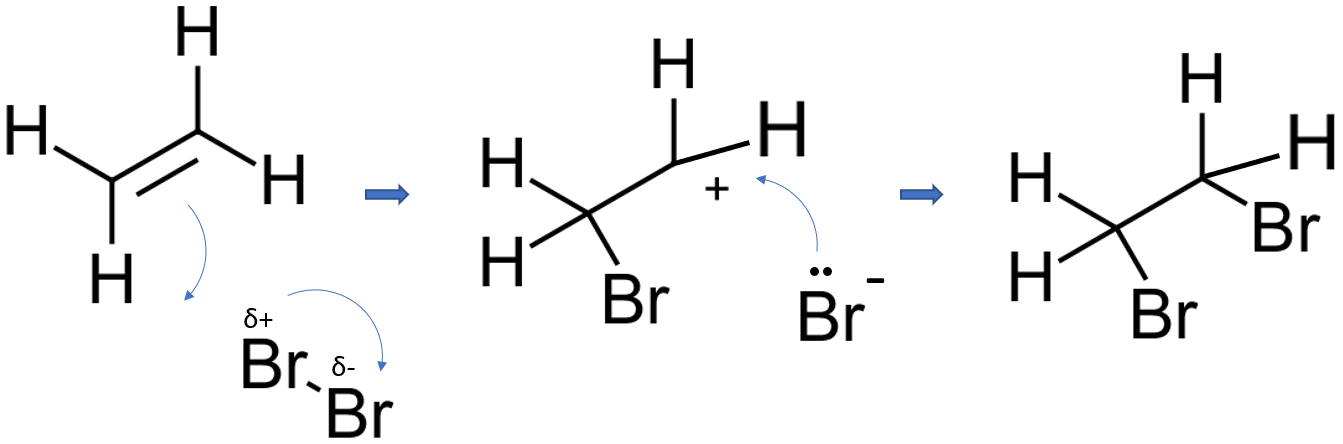
Suggested mechanism 2
In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. A bromonium ion is formed. The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction.