Complete and Incomplete Combustion
We could continue ad infinitum, showing the combustion of hydrocarbons and the production of Carbon Dioxide and Water. I will show one more, the complete combustion of Butane because this is quite relevant to anyone who has a gas heater, gas boiler et cetera in their house. The importance of regular servicing of these instruments cannot be overstressed because if they start to burn incompletely (usually characterised by a yellowy orange flame, instead of an nice blue one) they will start to develop Carbon deposits which will affect the efficiency of the heater/boiler, but more importantly they will start to produce dangerous gases such as Carbon Monoxide as well as Carbon deposits.

or

gives (generically)
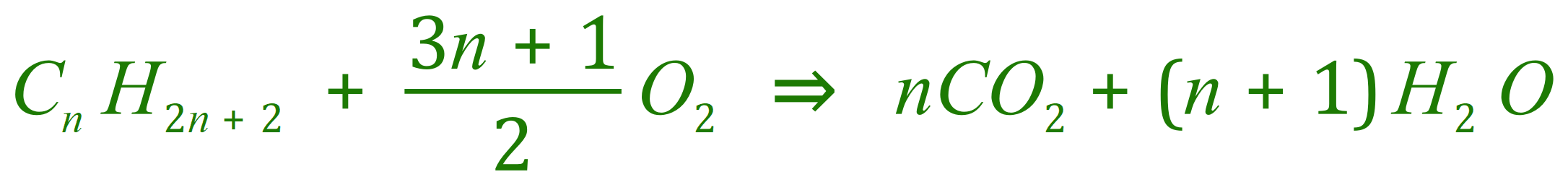
Take a look at the equation that we end up with, however if incomplete combustion starts to take place we start producing Carbon Monoxide and/or Carbon as well as Carbon Dioxide and Water:
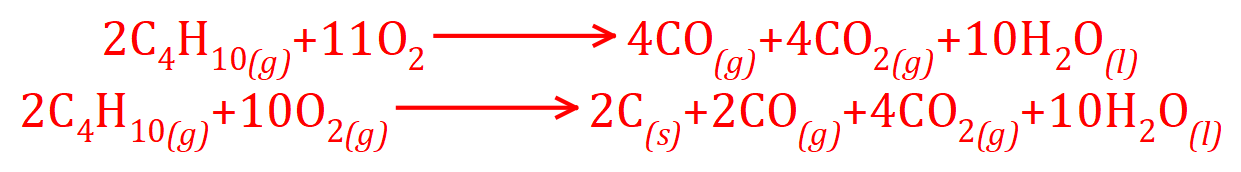
Clearly, there is insufficient Oxygen being used in the last two equations (11 and 10 mole) compared to the necessary 13 mole for a "clean" or "complete" burn.