Metallic Bonding
Metallic bonding is arguably the more straightforward out of the three you've seen so far. Unlike ionic bonding where electrons are "identifiably" transferred completely from one atom, or group of atoms to another, or covalent bonding where electrons are shared between atoms or groups of atoms, metallic bonding involves delocalised electrons.
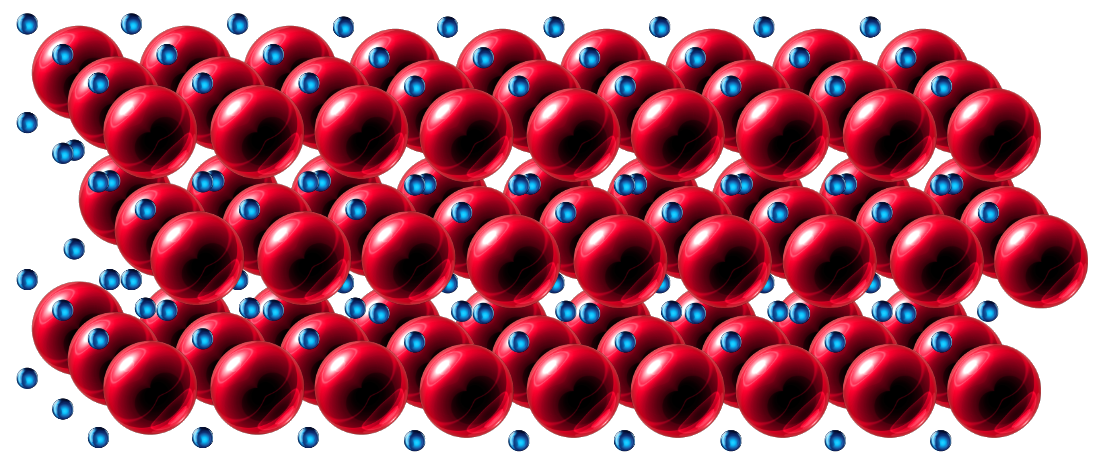
In metals, the electrons in the outer shells of the atoms are delocalised, that is they do not "belong" to any particular atom. This is often referred to in textbooks as a "sea of delocalised electrons" and as such can flow through the whole structure of the metal. If you look at the picture, try to imagine the deep red spheres as the positively charged metal ions eg: Zn2+ ions and the smaller blue spheres as the electrons, you can imagine any particular blue electron weaving its way around any of the red spheres in any of the three directions/dimensions, completely free and unrestrained.
There are strong electrostatic forces between the positive metal ions and negative electrons, these forces are what make up the "metallic bond" and this is what holds the structure together. The strength of the metallic bond accounts for the usually high melting points of metals, and the delocalisation of the electrons makes metals good conductors of thermal and electrical energy.
There are two properties of metals that should briefly be mentioned here, that of "Malleability" and "Ductility". A "ductile" metal is soft enough to be pulled into wires, one such example being the metal copper which can be drawn out into thin wires for use in electrical appliances etc.
A metal which is "malleable" can be beaten into thin sheets, hammered, rolled or otherwise forged into all sorts of different shapes. The reason for this is that the sheets/layers of atoms in the lattice can easily slide over each other. Take a look at the picture once again and try to imagine for example the top layer sliding left over the middle layer. One way to prevent this from happening is to add small amounts of other metals which contain larger or smaller atoms/ions. The sea of delocalised electrons will still be there but this time the "neatness" of the layers of the metal ions will be distorted by the fact that there will be the occasional atom/ion which is larger or smaller than its neighbours and this will disrupt the uniformity and neatness of the structure. As such the layers will now no longer slide across each other quite so easily and this has the effect of hardening the metal.
Metals which have been, if you like "deliberately rendered impure" by the addition of foreign metal atoms become "alloys". This additional hardness makes an alloy more useful than the pure metal. The most obvious alloy that we use every day is steel.