Electrolysis In Aqueous Solution
Let's consider perhaps one of the simplest electrolysis experiments that you might conduct, the electrolysis of Copper Sulphate solution.

The above picture shows an actual Copper Sulphate electrolysis setup being used. The electrodes are both graphite (carbon) and it can be seen quite clearly that the rightmost one is actively producing some sort of gas (in fact they both are, but the reaction on the left-hand side is somewhat slower).To be able to work out what these gases are requires knowledge of what ions we have in solution.
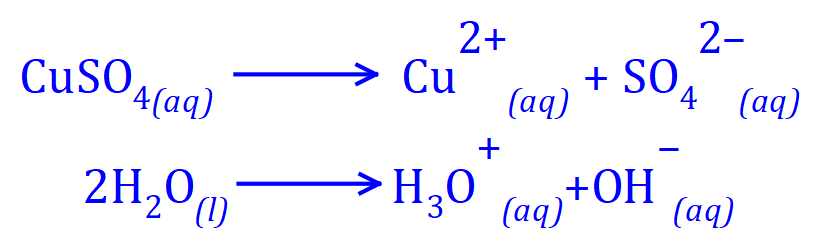
We must remember that as we are talking about "aqueous" solution we have to remember that as well as copper and sulphate ions will also have hydroxide and hydronium / hydroxonium ions.
So, in the electrolysis of copper sulphate in aqueous solution, what products can we expect?. At the positive electrode (which we know to be the anode) negative ions give up their excess electrons, being oxidised in the process. At the negative electrode (which we know to be the cathode) positive ions receive one or more electrons and are reduced to the base element. But wait, I can hear you say "this is all well and good, but we aren't any further forward, are we?".
Let's take a look once again at the reactivity series of metals.
|
|
In the reactivity series, the most reactive metals lie towards the top and the least reactive metals lie towards the bottom of the table. The positive ions we have are the Copper cation and the Hydroxonium ion. Out of the two, the least reactive is Copper and will therefore be displaced (reduced) to the metal. If this had been a solution of iron sulphate or zinc sulphate, the cation reduced would have been the Hydroxonium ion to Hydrogen gas. So what's happening at the other side? At the anode, negative ions which have migrated electrostatically that way will give up one or more electrons to be oxidised. What they are oxidised to depends on the choices available of the negative ion in the solution to start with. In our solution of Copper Sulphate we have hydroxide and Sulphate ions. Hydroxide ions will be oxidised to water and Oxygen gas. |

In general, if there is a halogen present in solution it will be oxidised to the element, if not then hydroxide will usually be oxidised to water, liberating Oxygen as shown in the equation above.
Let's take a look at the electrolysis of aqueous Sodium Chloride solution.

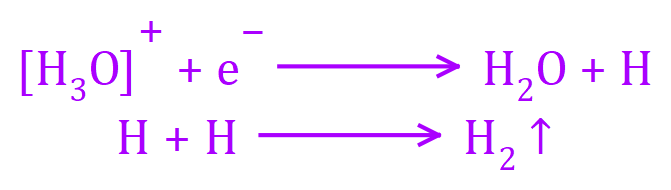
At the CATHODE:
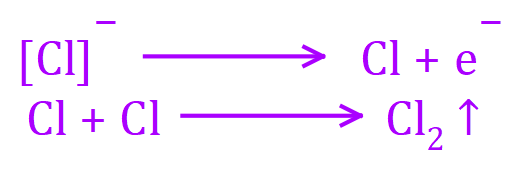
At the ANODE:
Go To >> Questions <<
