Electrolysis Of Molten Ionic Substances
The electrolysis of a molten Ionic substance is a little bit simpler because there are less ions in the mix to worry about. For example if we melt a sample of Lead Bromide and pass an electric current through it, the products of this electrolysis would be Lead and Bromine) :
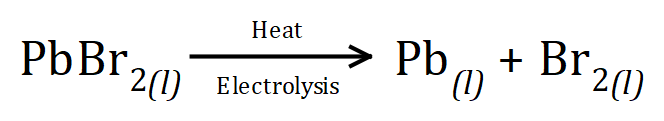
We only have Lead cations and Bromide anions to consider. As the positively charged Lead cations migrate towards the negative cathode they are reduced to elemental lead. At the positively charged anode, negatively charged Bromide anions are oxidised to elemental Bromine, atoms of which then covalently bond to form Bromine molecules.
The half equations are:
1. At the CATHODE:

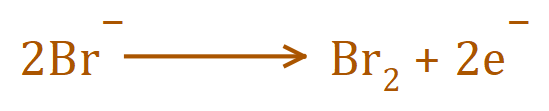
2. At the ANODE:
Go To >> Questions <<