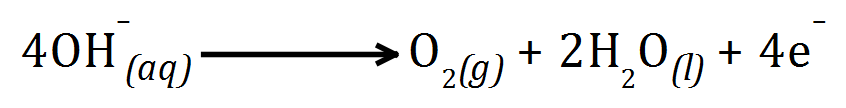Summary and Extraction of Aluminium
In electrolysis, ionic compounds are broken down into their component ions by passing electricity through the molten solution. The component ions either give up, or receive electrons to be oxidised or reduced to the elemental form.

Solid ionic compounds DO NOT conduct electricity because the ions are unable to move, they are locked in place. Molten ionic compounds DO conduct electricity because the ions are free to move. Aqueous solutions of ionic compounds conduct electricity for the same reasons as molten ionic compounds.
|
Cation - metal ION, one or more positive charges |
CATIONS MOVE TOWARDS THE |
CATHODE |
|
Anion - non-metal ION, one or more negative charges |
||
|
Cathode - electrode bearing the negative charge |
ANIONS MOVE TOWARDS THE |
ANODE |
|
Anode - electrode bearing the positive charge |
P.A.N.I.C. - POSITIVE ANODE NEGATIVE IS CATHODE.
The movement of IONS under the influence of an electrical current gives rise to either an OXIDATION or a REDUCTION reaction, depending on the ion and electrode:
Example: Molten Lead Bromide solution:
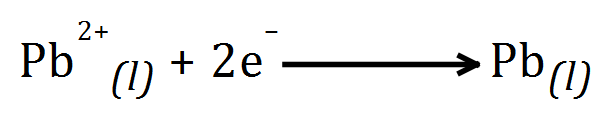
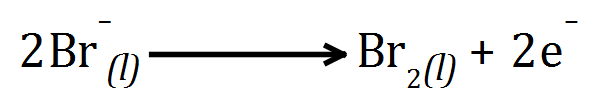
Lead cation is REDUCED to lead metal by accepting 2 electrons at the cathode. Bromide anion(s) OXIDISED to bromine liquid by giving up 2 electrons at the anode.
Example: Electrolysis of Bauxite / Cryolite mix (molten)
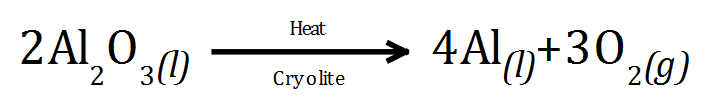
Cryolite is added to bring down the melting point of the mixture, this saves energy and makes the process more economical.
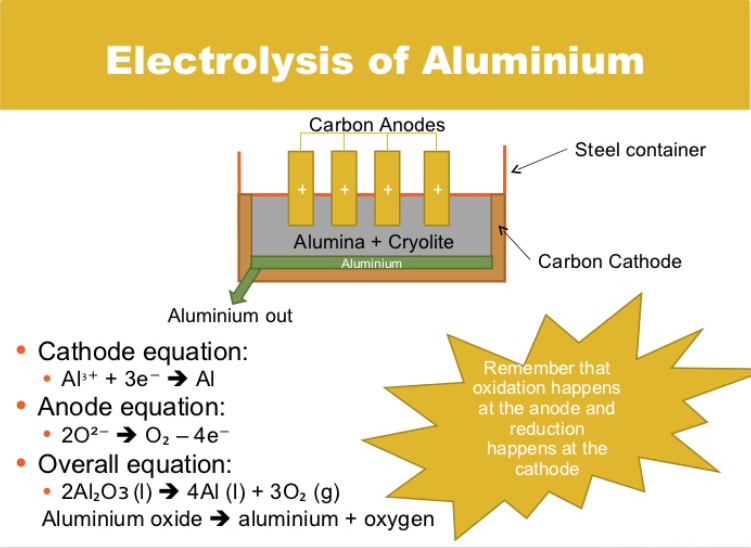
At the CATHODE:
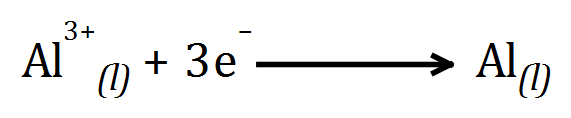
At the ANODE:
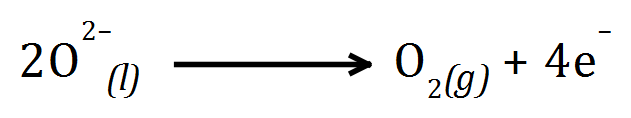
You may be asked:
Q. Why are the graphite anodes replaced occasionally after the electrolysis process?
A. The product at the anode is Oxygen gas, due to the high temperatures some of the gas reacts with the Carbon (making up the graphite electrodes) producing Carbon Dioxide gas which wears away the electrode:
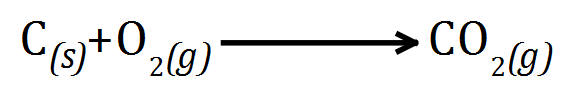
Electrolysis of aqueous solutions has to take into account the fact that there are 'extra' IONS floating around. This may have a bearing on what the products of electrolysis are.
Example:
Aqueous Sodium Chloride solution, the ions present are:
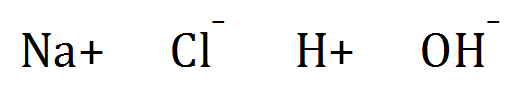
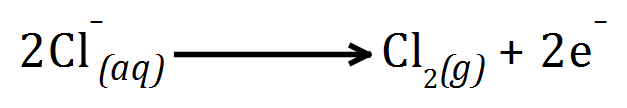
Generally where a halogen is present, it will be oxidised at the anode to the element, so the anode product will be Chlorine.
The product at the cathode depends on the element's position in the reactivity series of metals, as Sodium is more reactive than Hydrogen, it will be Hydrogen given off at the cathode by reduction:
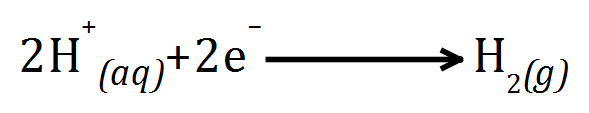
Where there is no halogen present, such as in the electrolysis of aqueous Sodium Sulphate solution, the product at the cathode will still be Hydrogen by reduction, however the product at the anode will be Oxygen gas by oxidation: