Nanoparticles
Nanoparticles are effectively very small pieces of material, the International Union of Pure and Applied Chemistry (IUPAC) has defined this as any particle with a diameter between 1 and 100 nm. Nanoparticles are usually made up of only a few hundred atoms and because this size falls below the lower limit of the visible electromagnetic spectrum, they cannot be observed with a light microscope. Nanoparticles have an enormous surface area to volume ratio and often display remarkably different properties to their corresponding bulk materials. To give you an idea of the scale of nano particle technology, look at the small table which gives approximate sizes of some readily recognisable every day items.
|
Atoms and small molecules |
0.1 nm |
|
Nanoparticles |
1 to 100 nm |
|
Fine particles (also called particulate matter - PM2.5) |
100 to 2,500 nm |
|
Coarse particles (PM10, or dust) |
2500 to 10,000 nm |
|
Thickness of paper |
100,000 nm |
Let us imagine a single nano particle, a cube with a side length of 100 nm which puts it at the top end of the accepted nano particle scale. What would its overall surface area be? What would it's volume be? and finally the ratio of Surface Area to Volume, which you will come across (unless you already have) in Biology when we talk about Exchange Surfaces and Diffusion.
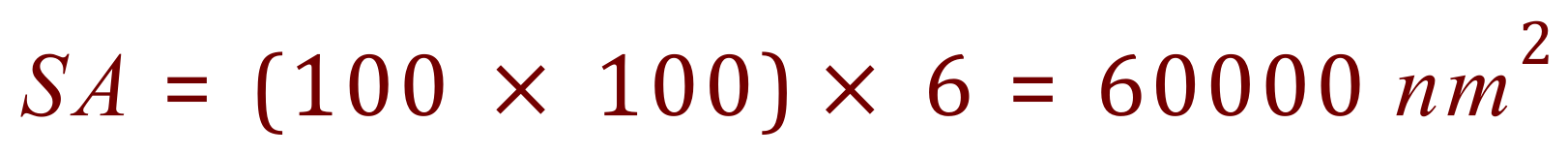
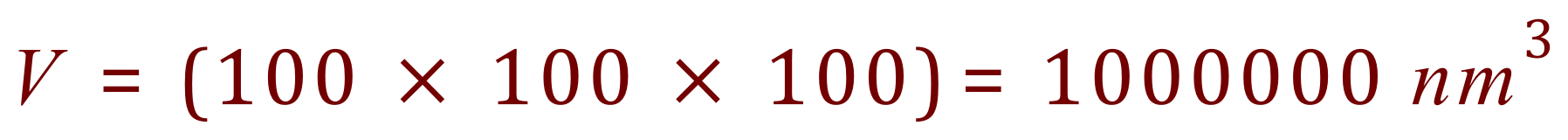
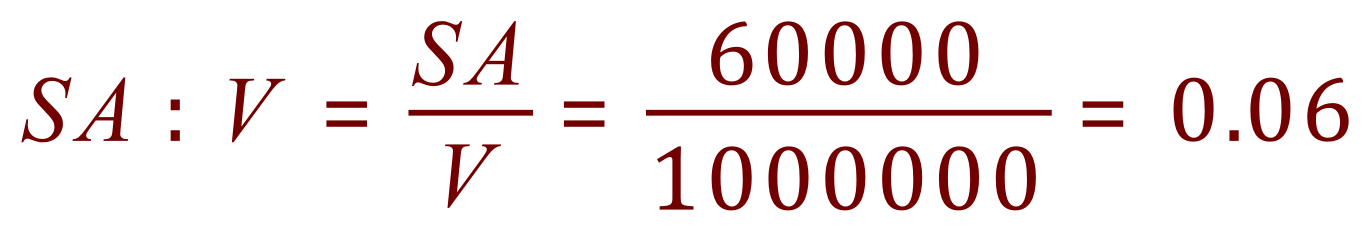
Now let us imagine taking a very small knife, and cutting the cube above into cubes of side length 10 nm, so effectively we have ten cubes wide, ten cubes deep and ten cubes high. We have cut our cube into 1000 smaller cubes!
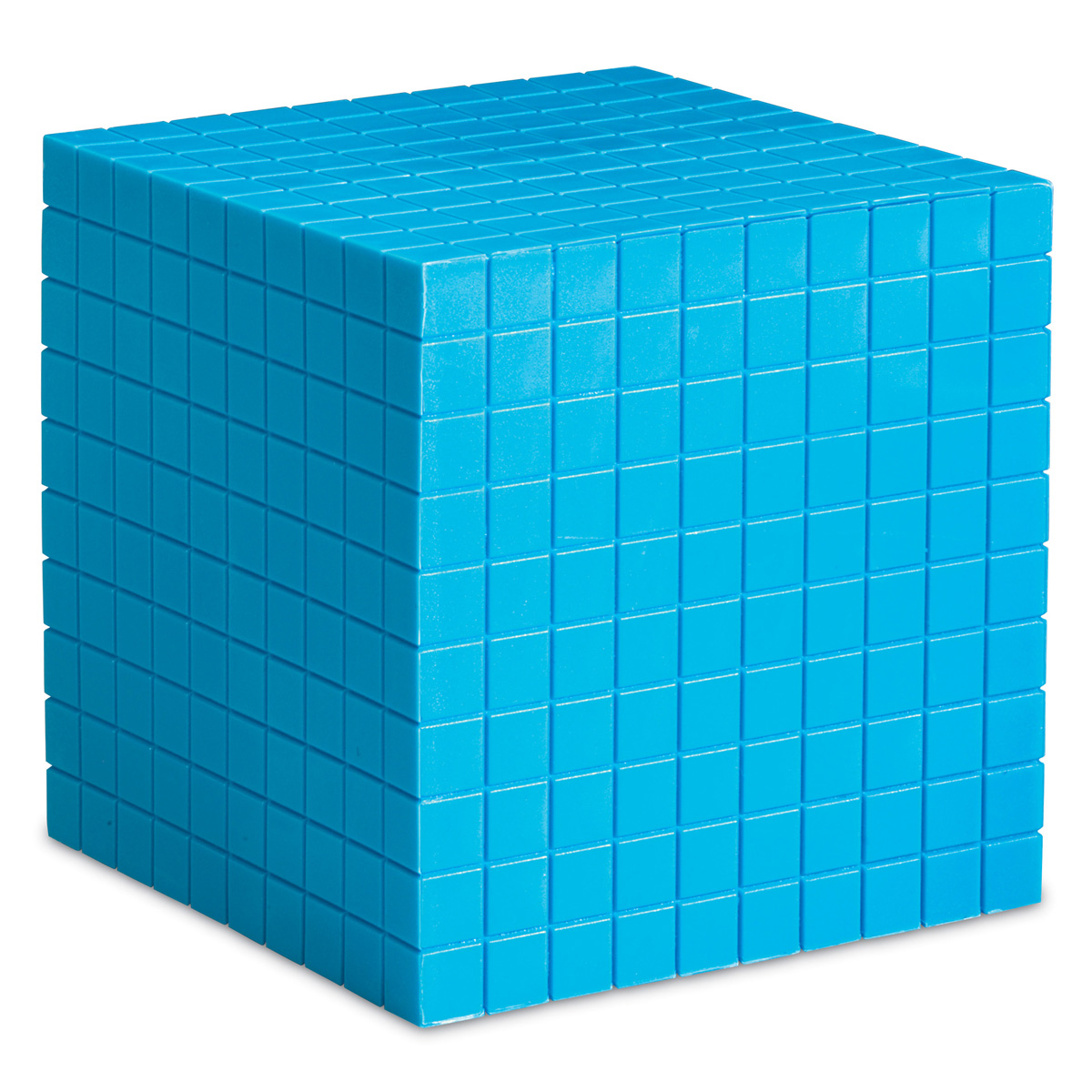
Much of this is already covered in the biology section on Exchange Surfaces, so I will show you the mathematics only.
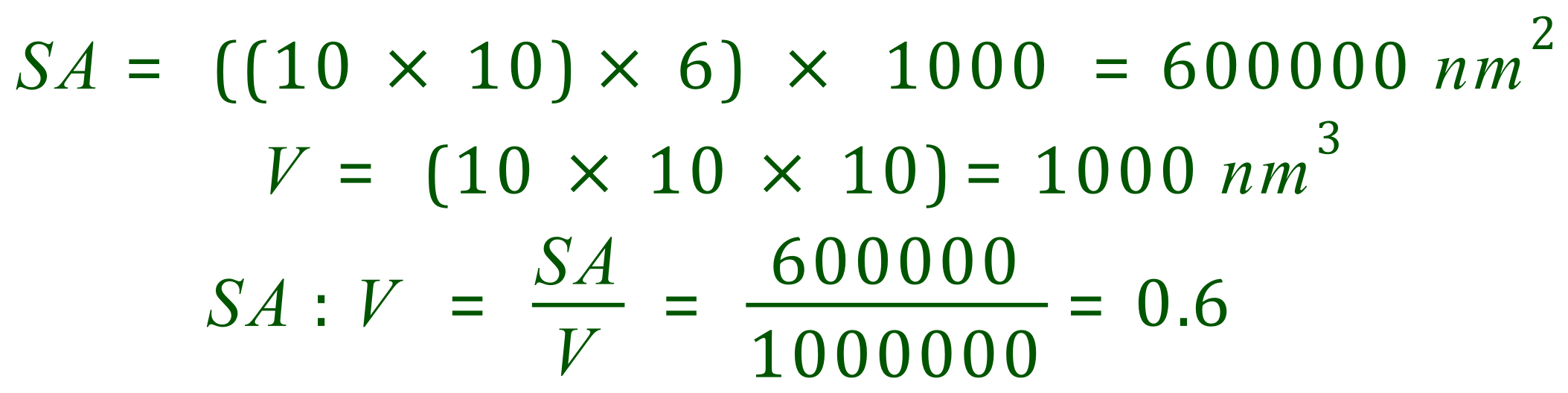
Decreasing the side length of the cube by a factor of ten (reducing it from 100 nm to 10 nm) has led to an overall increase in the surface area to volume ratio by a factor of ten. You will notice there is no difference in the volume, nor should there be, but the surface area increase is quite considerable.
https://en.wikipedia.org/wiki/Nanoparticle#/media/File:Colloidal_nanoparticle_of_lead_sulfide_(selenide)_with_complete_passivation.png (Accessed 13.04.2020)