Group 1 - The Alkali Metals
The alkali metals form group one of the periodic table. We have seen previously that the way in which we can tell which group an element belongs depends on the number of electrons in the outermost shell of the atom. The group 1 elements all have one single electron in their outer shell which makes them very reactive.
|
|
|
|
|
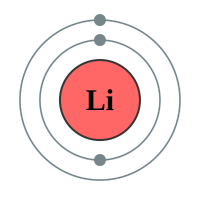
Lithium |
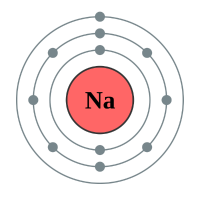
Sodium |
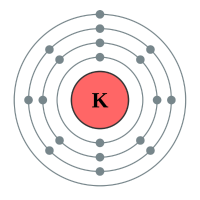
Potassium |
|
2 shells - 3 electrons - [2,1] |
3 shells - 11 electrons - [2,8,1] |
4 shells - 19 electrons - [2,8,8,1) |
|
|
|
|
|
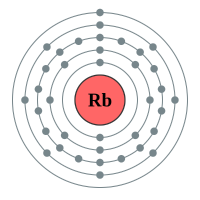
Rubidium |
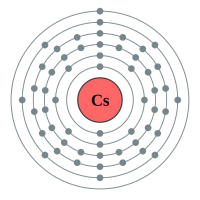
Caesium |
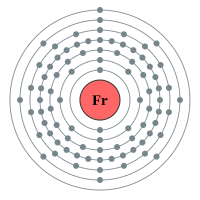
Francium |
|
5 shells - 37 electrons - [2,8,18,8,1] |
6 shells - 55 electrons - [2,8,18,18,8,1] |
7 shells - 87 electrons - [2,8,18,18,32,18,8,1] |
As far as we know all of these elements are silvery metal solids which react with water to produce the relevant metal hydroxide and release the gas hydrogen. They are so reactive the need to be kept away from water and therefore kept under oil.
The reactivity of the metal increases as you move down from Lithium through to Francium, but why is this?
Take a look at the six diagrams above, what do you see as you move from Lithium through Sodium Potassium Rubidium and Caesium and then on to Francium?
Lithium has two electron shells, Francium has seven electron shells. The attraction between the outermost electron and the nucleus of the Lithium atom is strong, the only thing getting in the way is the complete inner shell which contains two electrons. As we move to Sodium we see an extra complete shell of electrons between the nucleus and the outer electron. Moving on to Potassium we see yet another complete electronic shell between the nucleus and the single outermost electron. These completed shells of electrons have a "shielding" effect on the attraction between the outermost electron and the nucleus and what this does is effectively weaken the attraction between the two. As the attraction becomes weaker it becomes easier for the outermost electron to be lost, as a result the elements reactivity increases. This can be seen in experiments where Lithium is placed in water, producing a solution of Lithium Hydroxide and releasing Hydrogen gas. This is quite a slow, unimpressive reaction whereas if we replace Lithium with Sodium we see an increase in the reactivity, the reaction between Sodium and water is more impressive with the Sodium sample becoming so hot that it melts into a ball and floats around on the surface of the water.
By the time we reach Rubidium, the reaction is so violent that it cannot be conducted inside the laboratory and is usually done outdoors. There are many videos available showing the reactivity of Rubidium with water, and it is quite spectacular.
Q. The element potassium reacts violently with water, producing a solution of an alkaline salt and releasing a gas. Write out the word equation and then the balanced symbolic equation identifying the reactants, products and their states.
A. Potassium metal reacts with water producing an aqueous solution of Potassium Hydroxide and liberating Hydrogen gas:

|
Group 1 Metal / Element |
Melting Point (C) |
Boiling Point (C) |
Density (Kg/m3) |
Atomic Radius (nm) |
|
Li |
180 |
1342 |
535 |
0.15 |
|
Na |
98 |
883 |
968 |
0.19 |
|
K |
64 |
759 |
856 |
0.23 |
|
Rb |
39 |
688 |
1532 |
0.25 |
|
Cs |
29 |
671 |
1879 |
0.26 |
The table above shows some of the physical properties of the different alkali metals. You can see that as we go down the group from Lithium to Caesium the melting points decrease and the atomic radius increases.
There is a correlation between the two.
Group 1 elements have one electron in their outermost shell which is held very weakly by the nucleus. This electron can drift further from the nucleus than in most atoms of other elements. This results in Group 1 elements having larger atomic radii than those elements that follow them in their respective periods. The large atomic size results in weaker forces between neighbouring atoms. It is these weaker attractive forces due to the large atomic radii between neighbouring atoms of Group 1 elements that result in lower melting and boiling points when compared to other metals.
The decrease in melting and boiling points down the group can be explained by the additional shell being added to the previous element causing the atomic radius to increase. The increasing atomic radius means weaker forces between the atoms and so a lower melting and boiling point.
Q. Group 1 metals react with Water, Oxygen or Halogens to produce compounds. What sort of compound would you expect, and why?
A. The alkali metals react with these types of substances to produce ionic compounds. Ionic compounds are formed when an electron is transferred from one atom to another, as a result of which the element of losing the electron becomes positively charged and the element or group receiving the electron becomes negatively charged. In this particular case the entities of changed from being atoms to ions and as a result of which such compounds where the oppositely charged ions attract together electrostatically are known as "ionic" compounds.
The single outermost electron of the alkali metals is quite readily lost, therefore it is more favourable for the alkali metals to form ions rather than to bond covalently by sharing this electron.
Q. A 5g chunk of potassium metal is dropped into a trough full of distilled water, the resulting reaction produces a solution of potassium hydroxide and releases hydrogen gas. Assuming that water is "in excess" and that the reaction conditions are at RTP, calculate the approximate volume of hydrogen gas which will be released during this reaction.
A. To answer this question you need to do a couple of things, you need to know (and of course write down) the balanced reaction equation and have two hand some relative atomic mass data. If you need to go back and look at moles and molarity again, click >> here <<.
The balanced chemical reaction is as follows:

From the balanced equation you should be able to interpret that to moles of potassium will react with two moles of water to produce two moles of potassium hydroxide and one mole of diatomic hydrogen gas.
The relative atomic mass of potassium is 39 and the relative molecular mass of water is 18. The relative molecular mass of potassium hydroxide is (39+16+1) = 56 and the molecular mass of hydrogen gas is 2.
Now we have the numbers we can see that two moles of potassium would be 78 g, 2 moles of water would be 36 g, two moles of potassium hydroxide would be 112 g and one mole of hydrogen gas would be 2 g.
(You should be able to see from the law of conservation of mass that 78+36 = 114 and 112+2 = 114.)
We would end up with 2 g of hydrogen if we had started with 78 g of potassium, but in fact we started with 5 g of potassium. There are two ways now in which we could proceed with this calculation.
Method 1 - We know, or we can be told, or we can find out from a data book that one mole of any gas will occupy 24 dm cubed at RTP, so one mole of hydrogen gas (diatomic) would occupy 24 dm cubed. Taking this one step further, 78 g of potassium (2 mol) with excess water would produce 24 cubic decimetres of hydrogen gas.
We used 5g of potassium and would therefore produce:

Or approximately 1.56 dm cubed of hydrogen gas. The reason I quoted such a ridiculously long answer in the first instance is that you need to get used to not rounding until the very end of your answer. If you start to round off interim values in your calculations, you will ultimately throw out your final result. Always work to full calculator accuracy and quote your answer to the number of significant figures or decimal places that you are asked for at the end of the question.
Method 2 - Using mole values in the second method, we can first of all calculate of the number of moles of potassium metal we actually used. The relative atomic mass of potassium is 39 and so we in fact use:
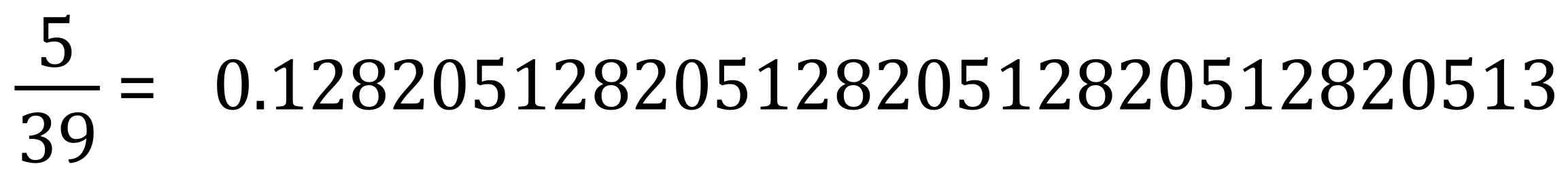
We use approximately 0.128 moles of potassium, but if we now look back at the equation we can see that the ratio of potassium used to hydrogen produced is 2 to 1, that is for every 0.128 moles of potassium we use we produce 0.064 moles of hydrogen gas. If you cannot see this, stop at this point and study until you're confident to proceed.
0.064 mol of diatomic hydrogen gas will occupy:
![]()
You should be able to see that the two results are the same.
Q. Which of the following alkali metals is the most reactive and why? (Potassium and Rubidium).
A.Out of the two metals given, Rubidium is by far the most reactive. The reason for this is that the outermost electron in the Rubidium atom is that much further away from the nucleus than its Potassium counterpart. The attraction between the nucleus and the outermost electron in Rubidium is much weaker than it is in Potassium, and as a result the outermost electron in Rubidium is more easily lost, this accounts for its greater reactivity than Potassium.
This change in reactivity can actually be observed in laboratory conditions.
The reaction between Lithium and water is not particularly spectacular, whereas Sodium reacts more violently and generates enough heat energy to melt the metal itself into a shiny silver ball, which skates quite readily across the top of the surface of the water. Potassium reacts even more violently, and the Hydrogen given off will ignite in most cases. The reaction between Rubidium and water and Caesium and water cannot be conducted in a laboratory as in both cases the reaction is quite explosive.
Back To >> [A] Oxidation State <<