The Structure of the Atom
One of the first things that you will learn in science, whether you study chemistry physics or biology but probably more relevant to chemistry and physics will be the structure of the atom. You will be told that the atom consists of three fundamental particles, protons and neutrons and electrons, two of which carry a charge, and one of them having no charge at all.
For the purposes of GCSE and probably a fair proportion of A-level study, this description will be satisfactory. The nucleus is made up of protons and neutrons, orbited at a relatively large distance from the nucleus by a number of electrons equivalent to the number of protons in the nucleus. The reason for this is that the protons carry a single positive charge each and the electrons carry a single negative charge each, these balance each other out make the atom electrically neutral.
The number of protons in the nucleus determines the element, for example in an atom of hydrogen there is one proton in the nucleus which is orbited (not strictly speaking the correct term, but it's how you'll be taught at this level) by a single electron. One positive proton is therefore electrically balanced by one negative electron and so the atom is overall neutral. In the particular case of hydrogen (or to be more correct, this particular isotope of hydrogen called protium) there are no neutrons. I had to make the distinction clear because in the next isotope of hydrogen, known as deuterium, there is a proton and a neutron however because the neutron carries no charge the isotope remains electrically neutral. In the third and final isotope tritium (which is radioactive) there is one proton, two neutrons, once again electrically neutral.
As a rule of thumb, and a good expression to remember, it is the protons that give the element its identity, but it is the electrons that give the element its characteristics and properties.
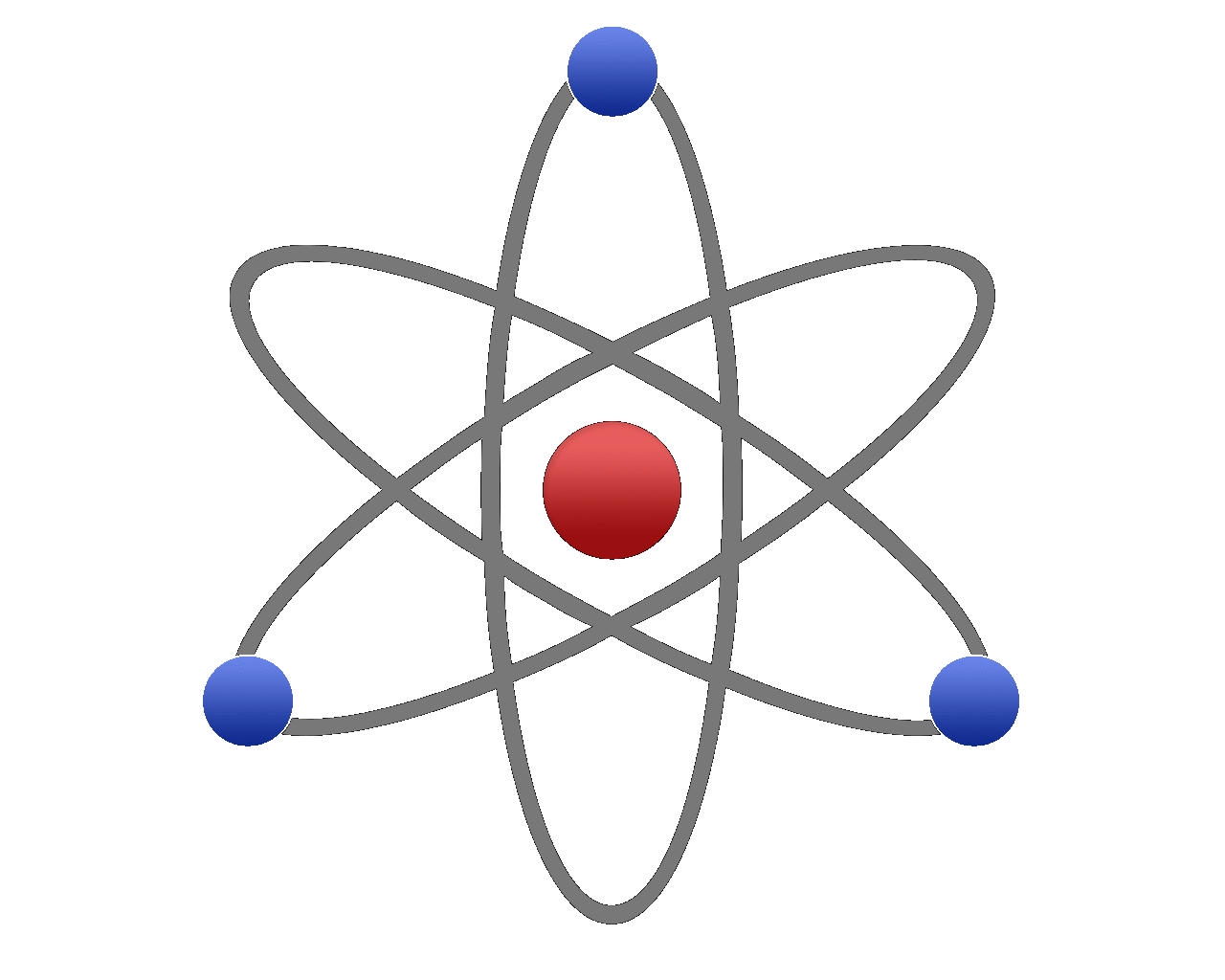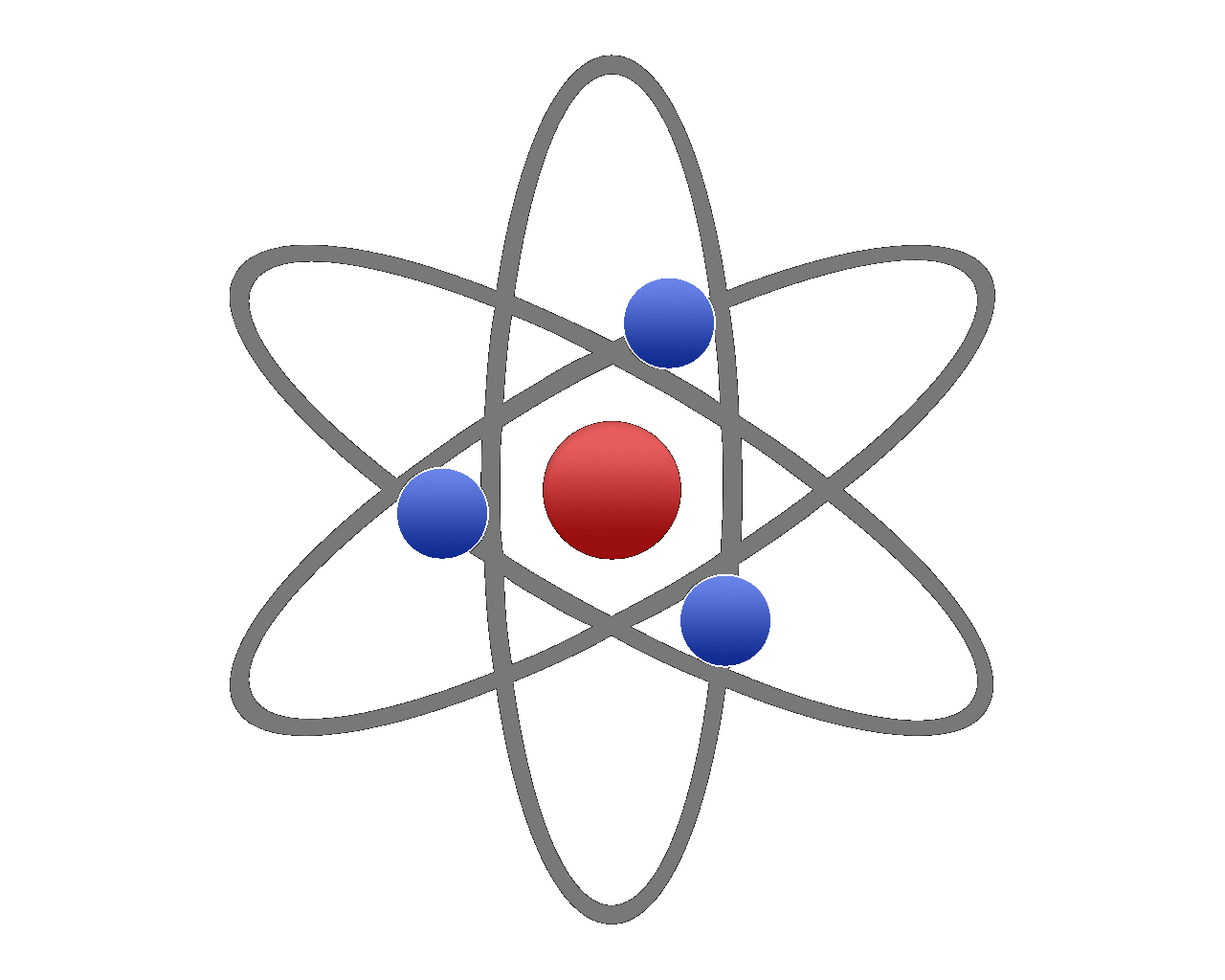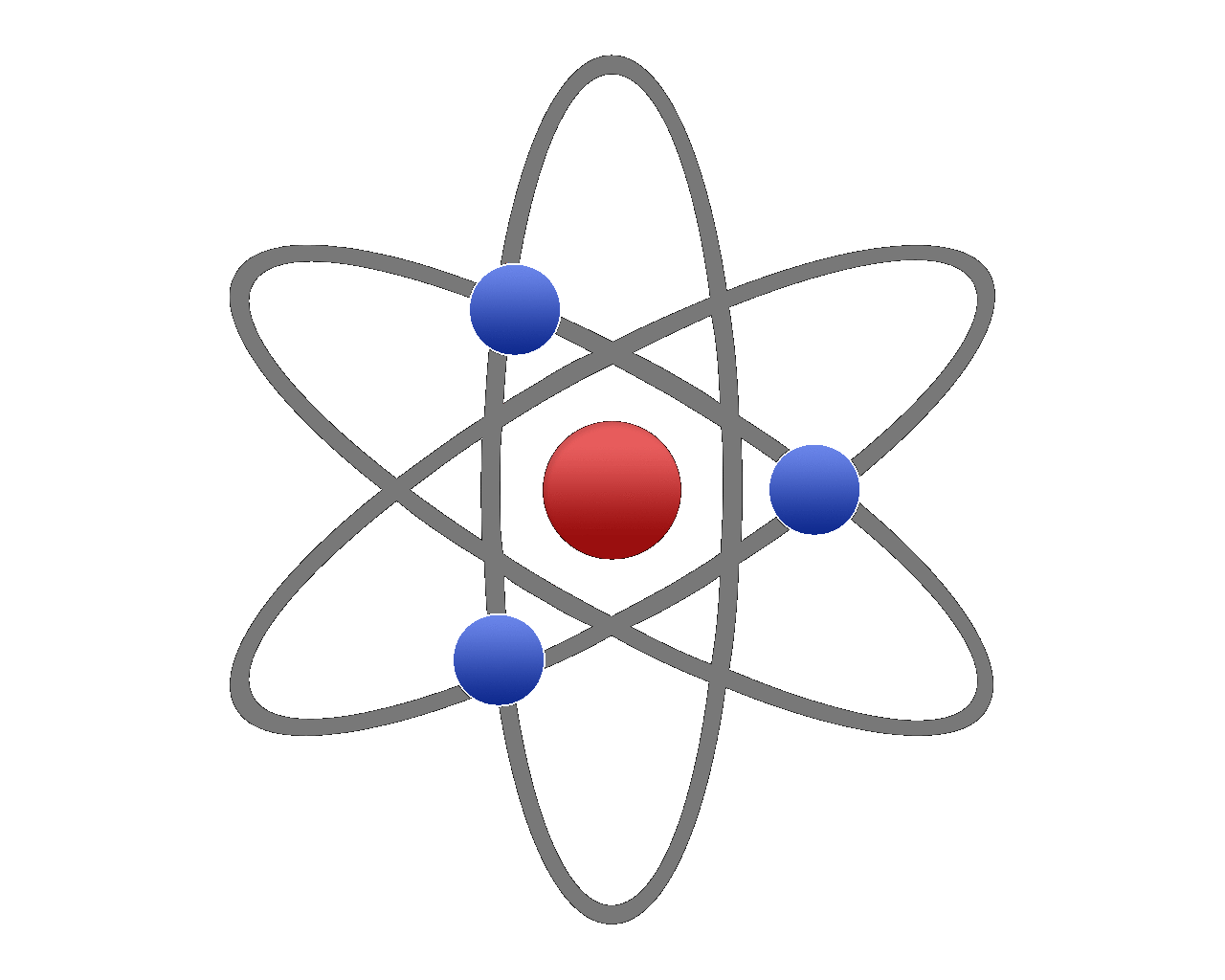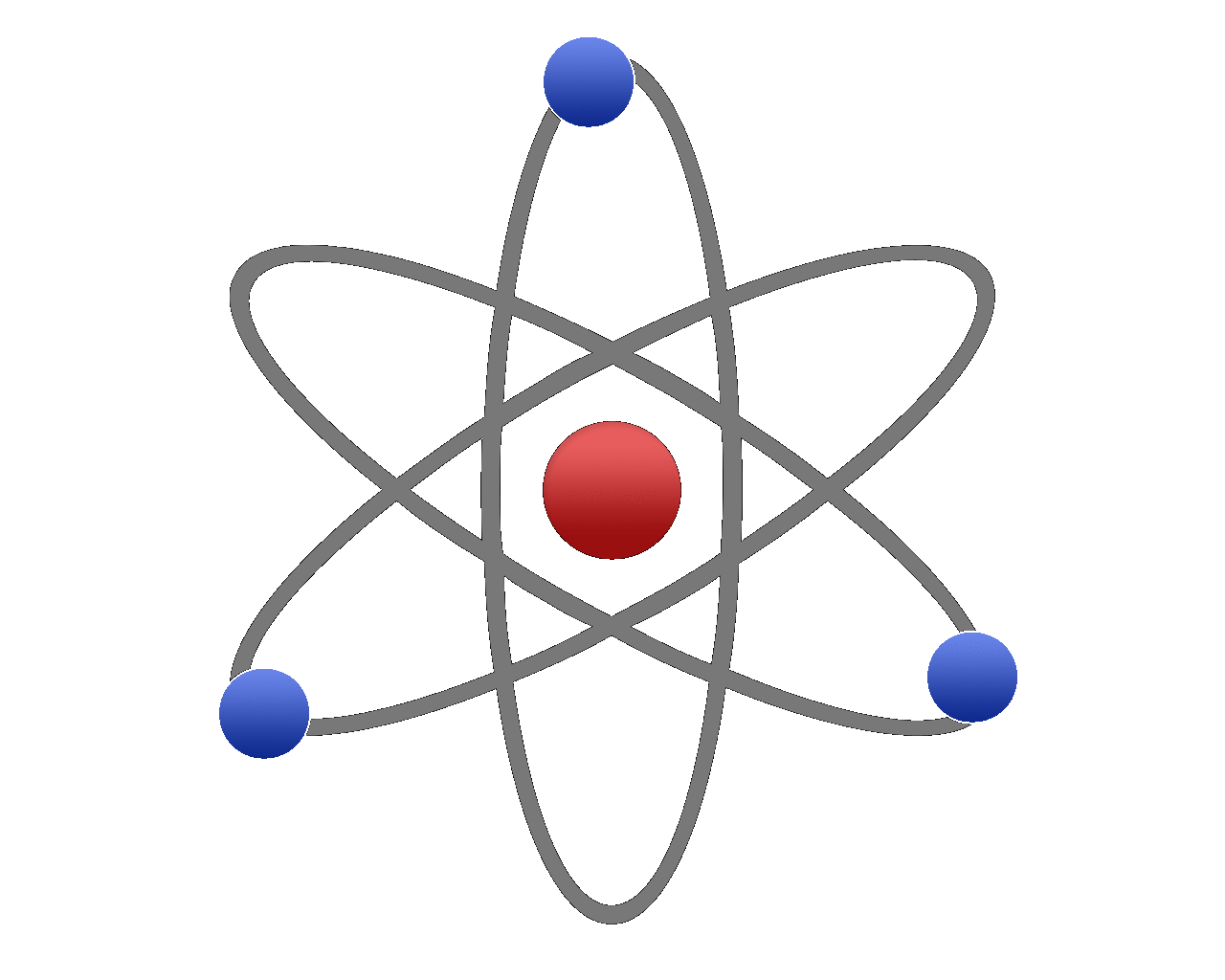
The picture shown is probably the way that you will be introduced to the structure of the atom, with the protons and neutrons making up the nucleus huddled together in a ball (and usually depicted as red for protons and blue for neutrons) with tiny electrons orbiting around this like the planets orbit the sun. The reality is quite different as this particular model is nowhere near to scale (for example the nucleus shown here is huge, but in fact it is quite small in comparison and the electrons orbit at quite massive distances away from the nucleus.
The atom is predominantly made up of empty space.
Q. Describe the structure of an atom. Use the terms for the subatomic particles Proton, Neutron and Electron in your answer.
A. The structure of an atom is as follows. The central nucleus of the atom contains sub atomic particles called Protons and Neutrons. Each Proton has a unit electrical charge which is positive, the neutron (as its name infers) is neutral. Orbiting the nucleus of the atom in energy levels, or shells, are the Electrons which have a unit negative charge. An atom is electrically neutral which means that the number of Protons and the number of Electrons will always be the same, each positive charge is cancelled out by the corresponding negative charge.
So what happens if an atom should gain a proton? Well it would become an atom of a different element, but unless it also gained an extra electron it would lose its overall neutrality and become a positively charged ion of the new element. For example if an atom of oxygen (which contains eight protons in its nucleus) were to suddenly gain an extra proton it would become a positively charged ion of the element fluorine. Only if the positive charge could be neutralised with an extra electron would it actually become a stable isotope of fluorine.
There is a lot more to consider than this, I am probably oversimplifying the matter but what I'm trying to do here is to show you that the number of protons sets the identity of the atom as I said previously. It is of course the electrons, and how they are lost or gained that will set the characteristics and properties of the element.
|
|
|
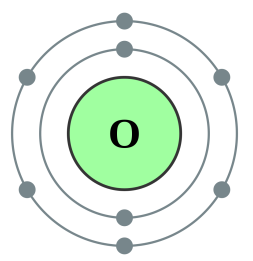
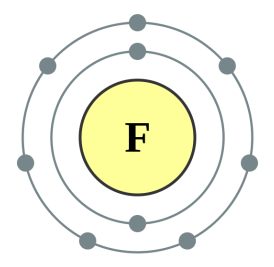
If you look at the picture of the oxygen atom above you will see that it is shown to have eight electrons, the content of the nucleus is not shown but will certainly have eight protons but potentially a varying number of neutrons depending upon the isotope. Similarly fluorine will have nine protons because as you can see it has nine electrons. There will be a varying number of neutrons, again because this will depend on which particular isotope of fluorine we have. In the scenario I described above we gained proton but we did not gain the extra electron, so we would in fact have a positively charged ion of fluorine, but nonetheless the identity of the atom would change.
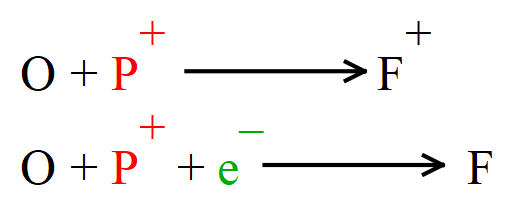
As I said previously, the structure of the nucleus consisting of simply protons and neutrons is an oversimplification and studying physics in particular to a deeper level will show you that the proton itself is made of different subatomic fundamental particles known as quarks, of which there are a number of different types. Protons can be produced from neutrons by the loss of a negative electrical charge and the emission of a certain amount of energy but these changes are way above the intention of this document and I won't go into them any further.