History of the Atom
So, we have seen a little bit about the atom and we are starting to gather a little bit of knowledge about what it contains and how this is all put together. However the structure of the atom, or the theories proposing the structure of the atom took quite some time to develop.
Back in 1897, scientist JJ Thomson performed experiments from which he concluded that atoms were not simply solid spheres as had previously been thought. His experiments produced results which suggested that an atom must contain even smaller negatively charged particles, "electrons" and therefore the notion of the atom being a simple solid sphere (like a ball bearing) needed to be looked at.
What could this be?
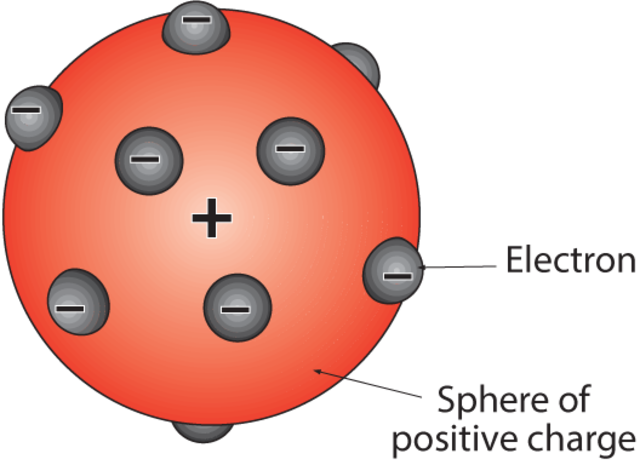
Well consider what the old pen picture of a Christmas pudding looks like, everybody sees a Christmas pudding as a brown ball with little black dots all over it covered (or at least half covered) in white icing with a sprig of holly on top. Well just look at part of this, the little brown ball with black dots which do in fact go all the way through and are randomly dispersed within the ball itself. This was the birth of the "plum pudding" model where the brown part of the ball was the atom and the little black bits were the negatively charged electrons.
Just a short 12 years later, in 1909, scientists Ernest Rutherford, supervising Hans Geiger and Ernest Marsden conducted an experiment whereby they fired alpha particles at a sheet of gold foil. If the "plum pudding" model was to be believed then the expectation was that the alpha particles would for the most part simply pass straight through or to be slightly deflected at most, but what they actually did observe was something quite different. Most of the alpha particles did in fact pass straight through, but some were deflected more than was expected and in some cases reflected backwards. This cast doubt on the accuracy of the plum pudding model and brought forward a new model where the atom was considered to be predominantly empty space (where most of the alpha particles would pass straight through) but with a point at the centre of the atom where most of the mass would be concentrated, and this mass (now known to be the nucleus) was the cause of some of the slight deviations (as the alpha particles are positively charged and the nucleus positively charged there would be repulsion) and in those cases where the alpha particles bounced backwards this would be as a result of a direct strike on the nucleus.
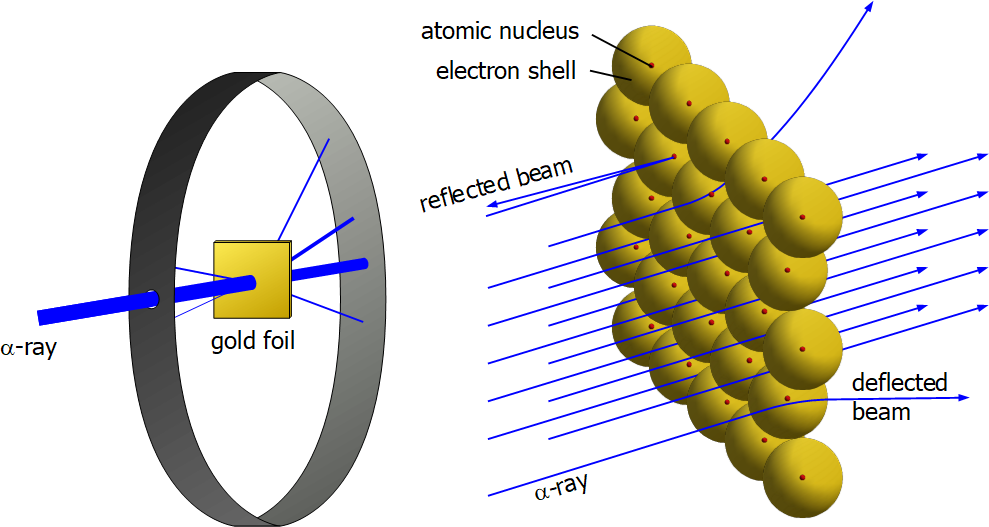
The electrons which were thought to be randomly scattered inside the mass were assumed to be surrounding the nucleus with most of the atom actually being empty space.
This Rutherford model was further enhanced subsequently by Niels Bohr who proposed that the electrons "orbiting" the positively charged nucleus would simply crash by electrostatic attraction into the nucleus (themselves being negatively charged) unless the electrons could only occupy certain energy levels around the nucleus, in other words the electrons were contained in "energy shells" orbiting the nucleus at fixed distances with nothing between them.
Analogy - think of this as walking up and down the stairs, if you are on the first step you need to move in "whole steps" to proceed upwards to the second, third, fourth and so on. Likewise to come down you have to come down, again, in "whole steps".
The picture here is an accepted representation of the structure of the atom at this level of study. Although the electrons do not in fact orbit as the planets would orbit around the sun it is a simple model to accept and only becomes more abstract and mathematically diverse as you progress towards A level and/or undergraduate level study.
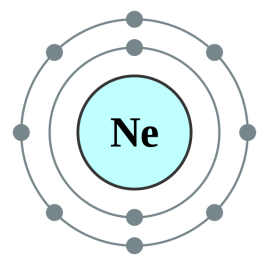
This representation (of a Neon atom) offers a simple explanation of the 10 electrons orbiting the neon nucleus, the innermost shell containing a maximum of two electrons and the outermost shell containing the remaining eight (which is in fact the maximum for the second shell, this will be dealt with subsequently).
Further experiments conducted by Rutherford and others led to the identification of the positive components of the nucleus as protons, subsequently scientist James Chadwick identified neutral particles residing in the nucleus along with the protons, which became known as neutrons. The discovery of protons and neutrons led to the development of the currently accepted model of the atom known as the "nuclear model".
Back To >> Radioactivity <<