Electronic Structure
We now know that atoms contain protons and neutrons and electrons. The protons and neutrons live inside the nucleus and the electrons orbit around the nucleus stop but exactly how are the electrons arranged in an atom?
Working on the accepted model of the atom put forward by Niels Bohr, electrons reside in "shells" or "energy levels" with the lowest energy shells being closest to the nucleus, and increasing in energy as we move away from the nucleus. The electrons fill up the lowest energy levels first, and once an energy level is complete any leftover electrons will move to the next energy level upwards. The real situation is a little bit more complicated than what is going to be described here, but at GCSE level this is the extent to which electronic structure needs to be understood.
The first energy level, or shall can hold a maximum of two electrons.
The second energy level can hold a maximum of eight electrons.
The third energy level can hold a maximum of eighteen electrons but we are only (at GCSE level) interested in the first eight.
[A] There is a simple relationship between the "principal quantum number" or "shell number" and the maximum occupancy it has:
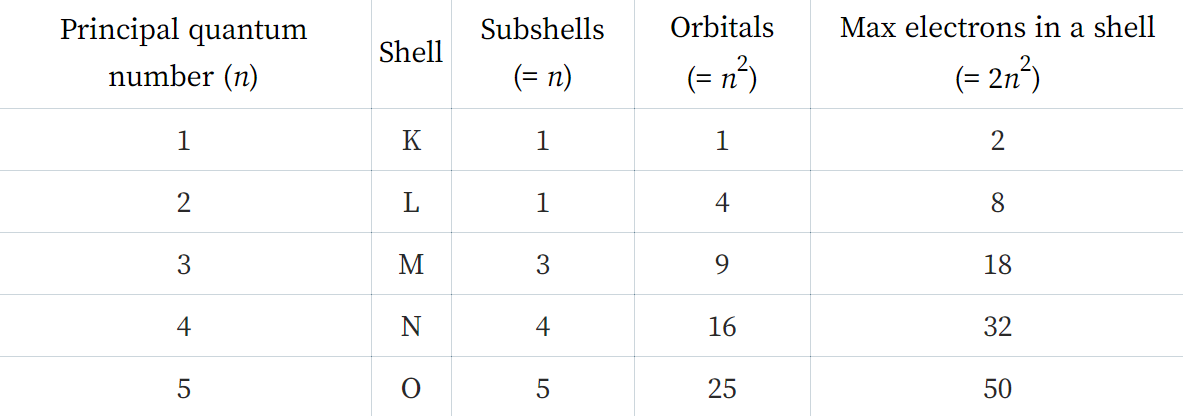
As we progress to fourth fifth and so on energy levels things become a little bit more complicated, we do not wish to concern ourselves with this at this time.
With the exception of the first shell, atoms tend to like their second and third shells complete with eight electrons (for reasons previously mentioned we will not worry about the fact that the third shell can in fact hold eighteen, we are only interested at this level in the first eight). Eight is a pretty unique number and will be referred to in many textbooks as the "octet rule". Atoms will generally do whatever they can to achieve the "full outer shell" either by losing or gaining one or more electrons. Let us take a look at one of the simpler atoms, phosphorus:
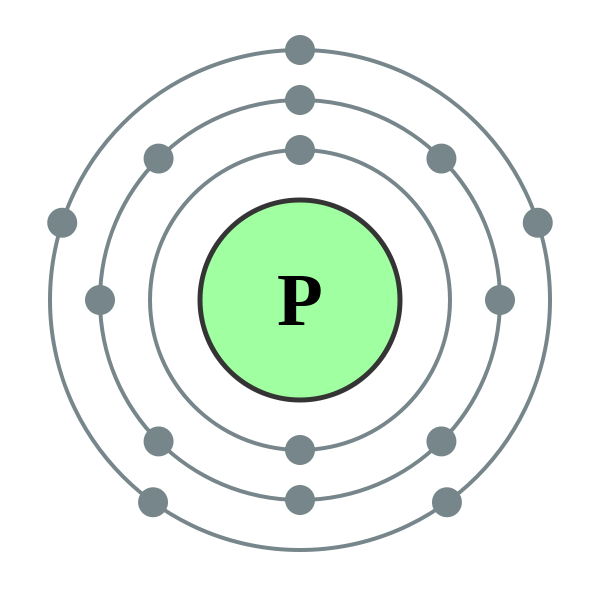
If we study the picture, we can see that the inner (lowest) energy level or shell holds its maximum of two electrons. If we take a look at the atomic number of phosphorus which is fifteen we automatically know that a neutral atom of phosphorus will also contain fifteen electrons, so where do the other thirteen go? Well, we said that our second shell will hold a maximum of eight so if we put eight of the thirteen into the second shell that leaves us with five and this remaining five stay in the third energy level as can be seen in the diagram. The electronic structure of phosphorus would therefore be written 2,8,5 or [2,8,5]. It is the outermost electrons that we are interested in in chemical bonding, these are the "valence" electrons.
Q. Using the model above, write out the "square bracket" notation for the electronic strictures of the first ten elements.
A.
|
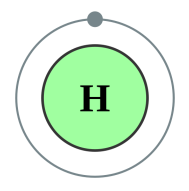
Hydrogen [1] |
Helium [2] |
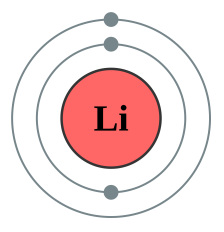
Lithium [2,1] |
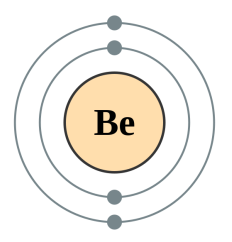
Beryllium [2,2] |
Boron [2,3] |
|
Carbon [2,4] |
Nitrogen [2,5] |
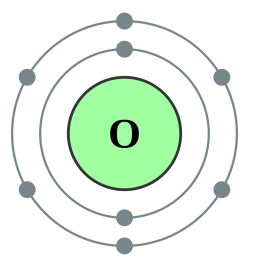
Oxygen [2,6] |
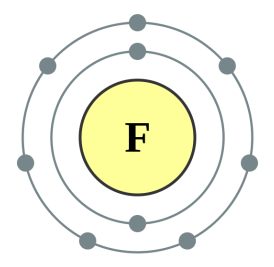
Fluorine [2,7] |
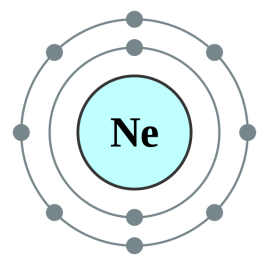
Neon [2,8] |
|
|
|
|
Hydrogen [1] |
Lithium [2,1] |
|
Group 1 |
Group 1 |
|
Period 1 |
Period 2 |
|
|
|
|
Oxygen [2,6] |
Fluorine [2,7] |
|
Group 6 |
Group 7 |
|
Period 2 |
Period 2 |
|
|
|
|
Beryllium [2,2] |
Neon [2,8] |
|
Group 2 |
Group 8 |
|
Period 2 |
Period 2 |
In the table above I have obtained six further electronic structures of various elements. In particular I have stated also the group and period on the periodic table where each element would be found. Take a good look at the electronic structures, pay particular attention to the number of electrons in the outer shell, the total number of shells and see if you can spot any patterns?
Okay, if you'd studied this long enough you should have spotted that:
- An elements group is determined by the number of outer electrons
- An elements period is determined by the number of energy shells
So taking our top example of phosphorus we can see that there are five electrons in the outer shell and three energy levels therefore phosphorus is a group of five element in period three.
Here is an element....................Calcium:
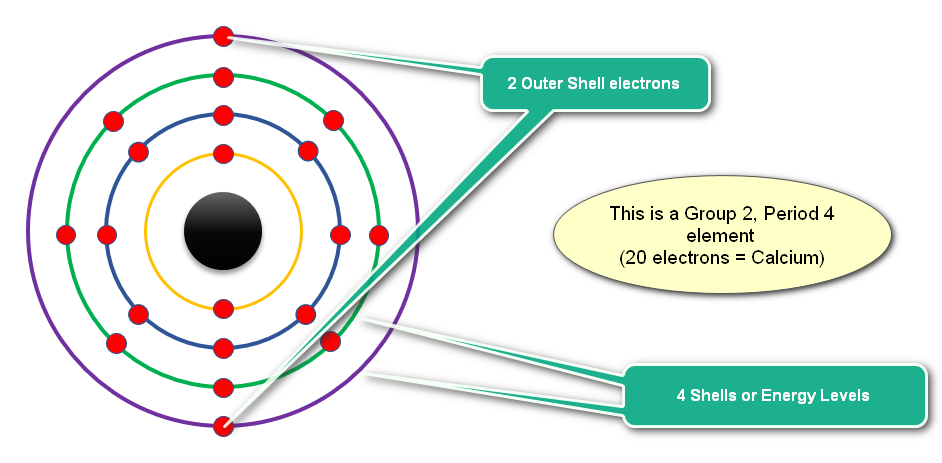
>> First 20 Element Configurations <<