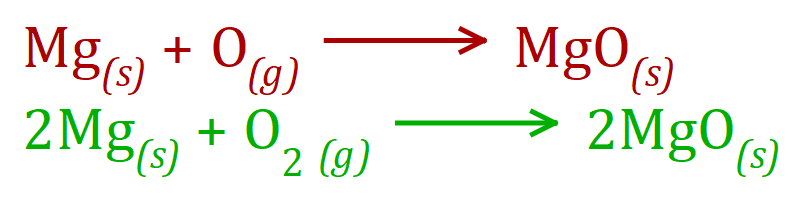Valency
It is the OUTER shell of ELECTRONS in the ATOM which take part in chemical bonding. The number of outer electrons sets the GROUP number and the number of shells of electrons sets the PERIOD of the Periodic Table that the element is found.
In the case of Magnesium you can see that there are 2 electrons in the outer shell and 3 shells, so we say that Magnesium is found in Group 2, Period 3 of the Periodic Table.
It can help if you think of VALENCE ELECTRONS as HOOKS (generally in metals) and VACANCIES in the outer shell (generally in non metals) as EYES.

So, a metal with hooks can link up with a non metal with eyes, in the amounts determined by how many of each they have. Lets take a look at an example to see if we can clarify this a little.
Take Calcium and Chlorine, if you look at the PTE you will see that Calcium has “2 Hooks” and Chlorine has “1 EYE”.
Q. How many Chlorines could a Calcium atom link up with?
Q. What PERIOD and GROUP do these elements occupy?
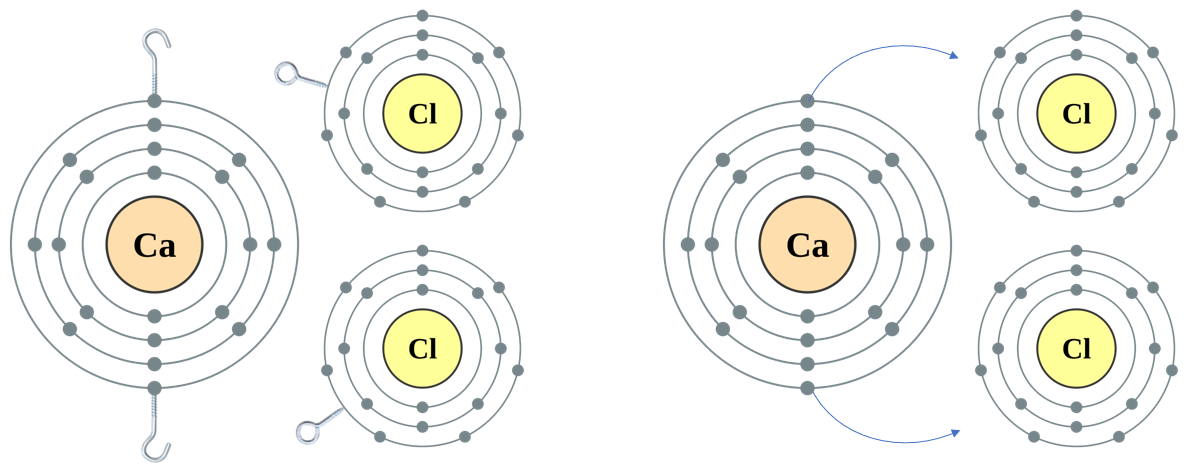
A. Calcium has “2 Hooks”, Chlorine has “1 Hook”, so we can link TWO Chlorines to ONE Calcium.
A. Calcium is in Group 2, Period 4 and Chlorine is in Group 7 (1 “Eye” less than the stable 8 electron shell) Period 3.
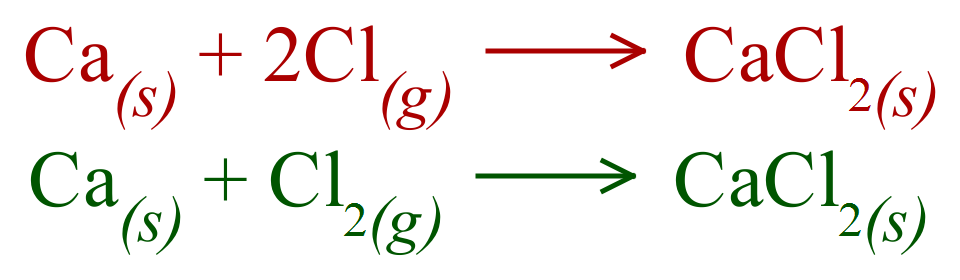
Q. Take a look at Sodium Bromide:
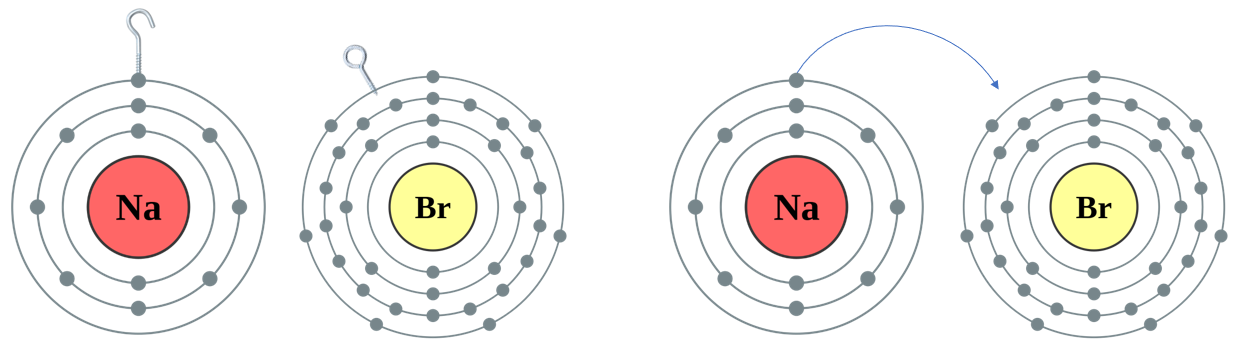
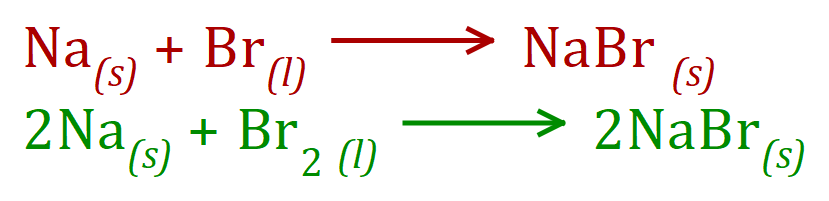
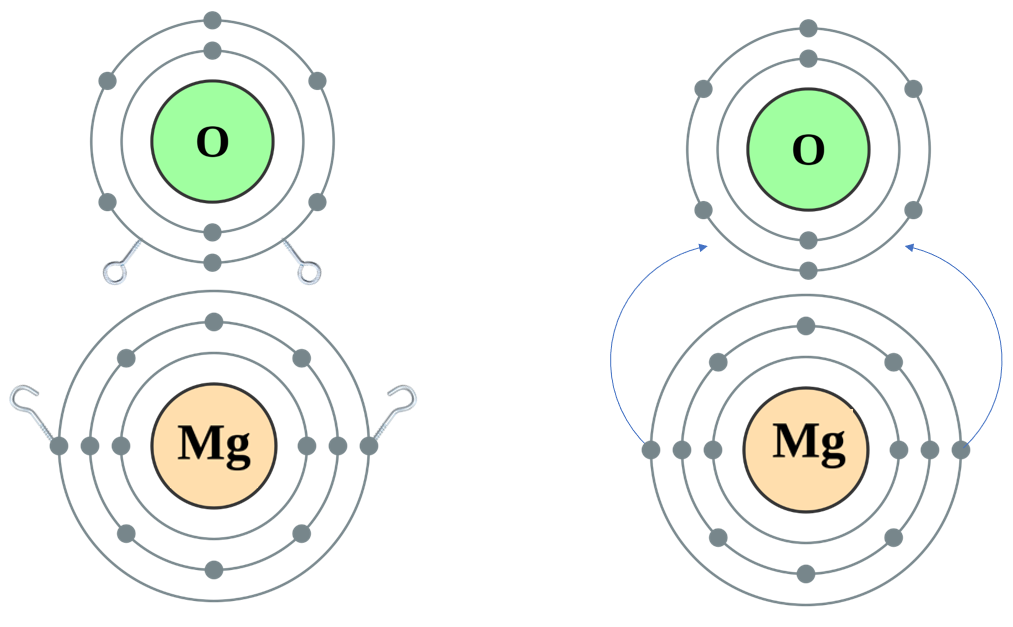
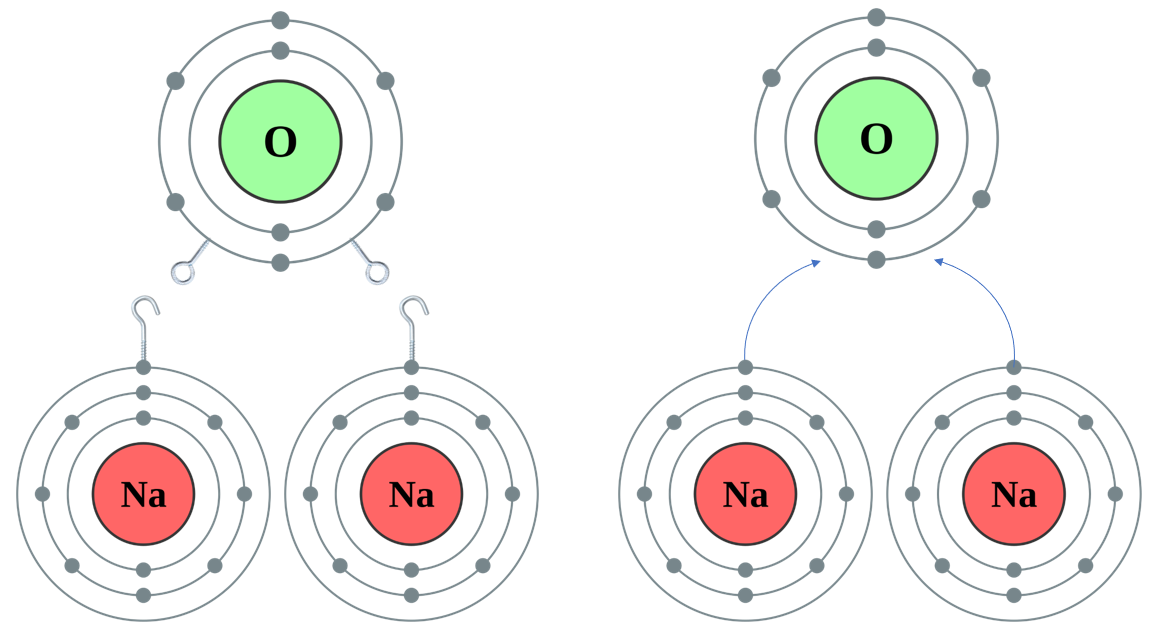
Now ..... let us have a look at a situation where the metal has only "1 Hook" but the nonmetal has "2 Eyes", as in the case of Sodium Oxide:

And now, a situation where there are "2 Hooks" AND "2 Eyes" - Magnesium Oxide: