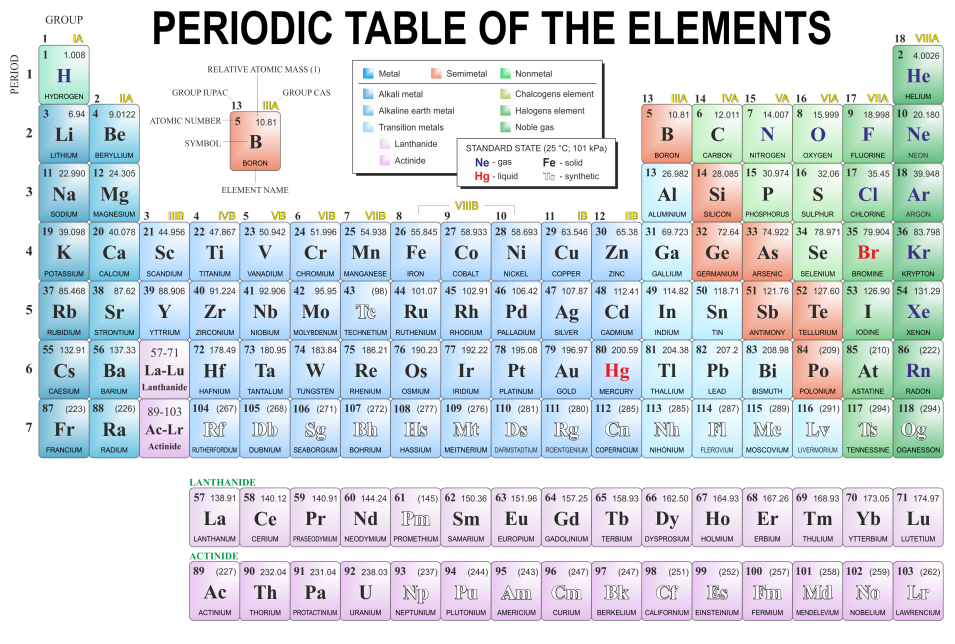
The Periodic Table
The periodic table is a table which contains a listing of all of the known elements. Initially put forward by Dmitri Mendeleev (1834 to 1907), this version of the periodic table came about by Mendeleev taking earlier versions and rearranging all of the known elements and arranging them according to atomic number, but when this didn't fit with the known properties, swap rounds were made.
A clear example of this, if you study the periodic table is the element tellurium and the element iodine, since tellurium has the larger relative atomic mass but properties dissimilar to the elements in the same group as iodine. It was found that iodine more properly belonged in what we now know to be group 7 as its properties were similar to elements already located in that group.
In these early days, scientists had no notion of protons, neutrons or electrons. Elements were arranged by the one thing that they could be measured by, their relative atomic mass and when this was done, scientists started to notice patterns developing in the properties of the elements. As a result of this, the elements were grouped according to these properties with gaps being left where the properties didn't agree, suggesting that new, as yet undiscovered elements would be located and would fit in the appropriate places.
Click on the PTE map, the coloured areas representing groups or periods will take you to the relevant section (where hyperlinks have been set).