Groups and Periods
As a result of the work of Dmitri Mendeleev, the modern periodic table was born where the elements are arranged in order of increasing atomic number and grouped according to properties.
Elements displaying similar properties were placed into vertical columns which became "groups" and running across the table, these arrangements became known as "periods". An element can therefore now be characterised by each group and period setting its position in the modern periodic table.
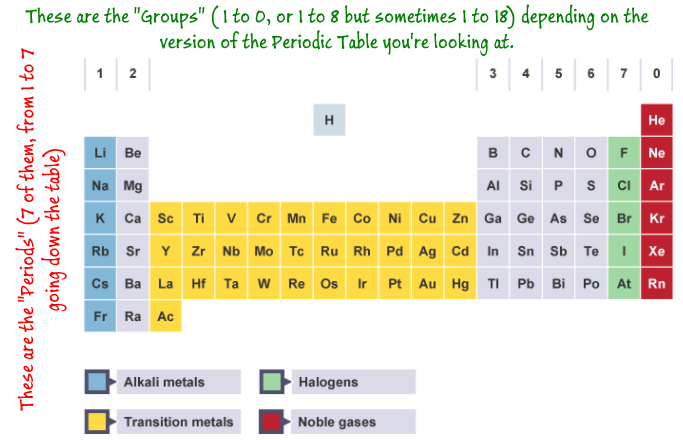
Period 1 is the row with H and He, and continuing down to Period 7 we have the row containing Fr to Og and the Actinides.
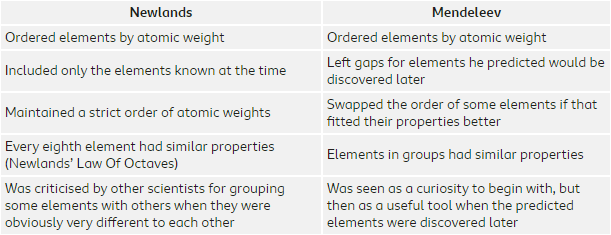
So, who decided that the periodic table would look like this? Well, back in 1864 there weren’t as many elements known as there are now, but an English scientist named John Newlands arranged all of the known elements in order of their atomic number (the number of protons in the nucleus) as he noticed that there were similarities between certain elements and of those eight places in front.
This system didn’t last very long, because Newlands ended up having to put some elements in places in his periodic table, where their properties did not match what would have been expected. Newlands version of the periodic table wasn’t widely accepted, it was only when Dmitri Mendeleev’s table came along five years later, in 1869 that the periodic table that we now know started to be developed.
Just like Newlands, Mendeleev put his periodic table into atomic number order, but also matched the elements in vertical groups according to their properties. This meant that there were gaps, but Mendeleev simply regarded this as elements which were yet to be discovered. Not only that, Mendeleev went on to predict the atomic numbers and properties of these elements even though they hadn’t yet been discovered, but when they were it was shown Mendeleev was right.
Although the periodic table put forward by Newlands was quickly replaced, his “law of octaves” was very interesting, and the number eight features quite prominently when we come to look at the way in which electrons are arranged in an atom.
Very briefly, an atom has exactly the same number of electrons as it does protons. This is because electrons have a single negative charge, and protons have a single positive charge. When these charges meet, they balance each other out very much like putting the same number of items in each pan on an old set of weighing scales. When the scales balance it means that one side is equal to the other. In the case of protons and electrons the “balance” occurs when the charge overall is zero, in other words the atom is neutral.
We visualise electrons orbiting the nucleus of the atom just like the planets do around the sun. It’s not quite that straightforward but at GCSE level it is a useful model. The “orbits” get larger as we move further away from the nucleus, just like the orbits of the larger planets in the solar system the farther they get from the sun.
The first “orbit” or “level” is closest to the nucleus, and can contain a maximum of two electrons. If you consider the first two elements Hydrogen and Helium, you will see that very quickly this first level becomes full.
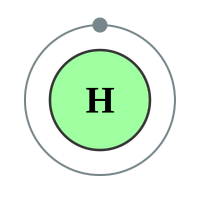
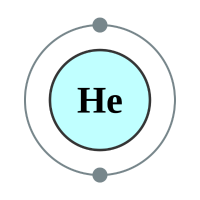
The next element is Lithium which has three protons and therefore three electrons. Now if the “level” can contain only two electrons, where do we put the third one? Well we have a second shell further out from the nucleus than the first one, and we put it in there. Now this second shell is different from the first because it can contain eight electrons (remember Newlands octet or law of octaves?). We can continue adding electrons to the shell until it is full, when you reach Neon.
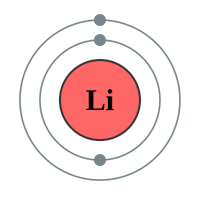
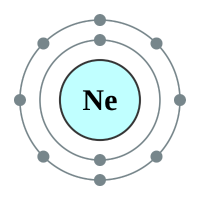
Now, because Neon can hold eight electrons in its outer shell, and has got that number, it is quite happy to stay that way and will not accept any more electrons. It is electrons that determine the reactivity and properties of the element, so because Neon is happy with what it’s got, it’s not reactive. What happens if a ninth electron comes along? Well once again we make another energy level (or shell) and we put it there. Welcome to the element Sodium:
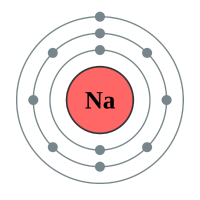
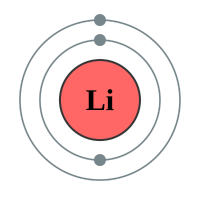
Now I have put Lithium next to Sodium for a particular reason. Can you see a similarity between the electronic configuration of Sodium and that of Lithium? I’ll give you a hint, have a look at the outermost level, how many electrons are there?
There is a saying in science:
“it is the protons that give an element its identity, but
it is electrons that provide its character!”
This is very true, the number of electrons and how available they are to take part in reactions will determine some of the properties of the element.
You should have spotted that the outer level of each element has got just one electron, and the element will happily use that electron in chemical reactions. How reactive the two metals above, which both coincidentally fall into group 1 the periodic table, will be depends on how easily the atom will let go of that outermost electron.
Let’s quickly take a look at some magnets:

If you try this experiment with two magnets, start with them at the furthest distance, perhaps just pushing the one towards the other and you will find that depending on the attractive force (in this case how strong the magnets are) at some point they will jump together.
Now remember that this is an analogy, simply a model to try to explain
the attraction between electrons and protons in an atom.
Just to make it easy for you, I have repeated the diagram of Sodium and Lithium with the next member of the group, Potassium:
Q. Which nucleus will have the greatest “pull” on its outer electron? Potassium, Sodium or Lithium?
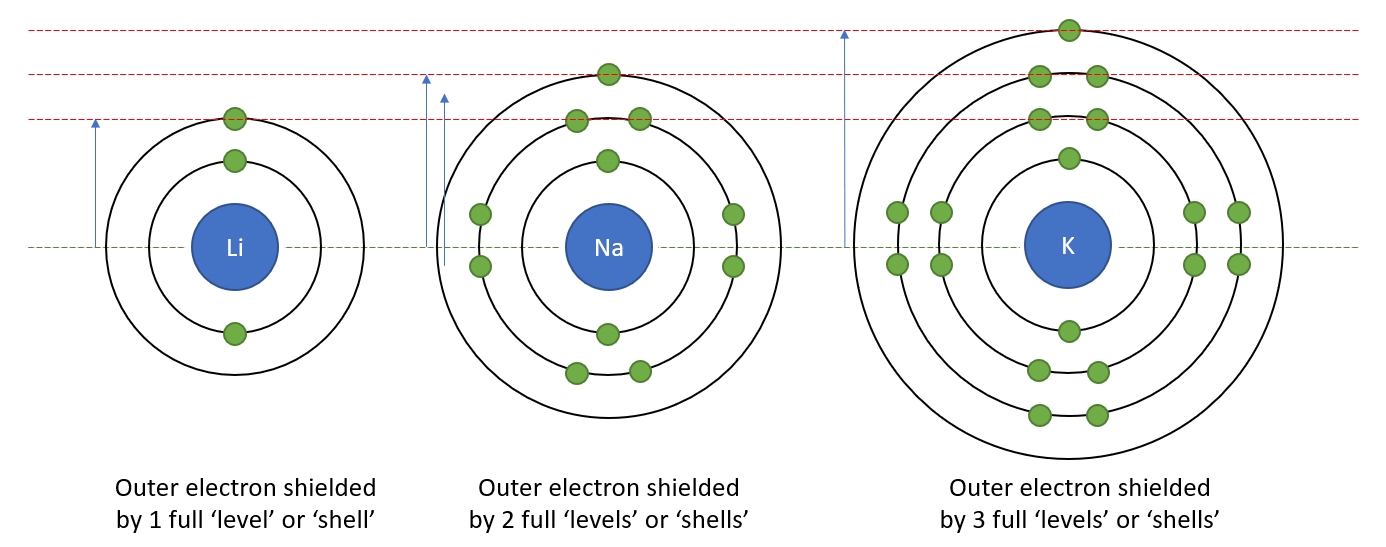
A. You should have concluded that Lithium’s nucleus will have the greatest pull on the outer electron because they are closer together than the Potassium or Sodium nucleus and its outer electron. In addition to this Lithium only has one full level between its nucleus and the outer electron, whereas Sodium has two and Potassium has three. This will produce a shielding effect which will weaken the attraction between the Sodium (Potassium) nucleus and the outer electron as well as the fact that they are further apart because the atom is larger anyway.
The easier it is for an atom to lose (or gain) and electron, then generally the more reactive that atom will be.