[A] Radioactivity
When we speak about a substance being radioactive, we take this as the substance emits or gives off radiation. You probably already know that certain radioactive elements used in college laboratories are kept in lead lined wooden boxes, they are retrieved for the teacher to give you the lesson on radioactivity and radiation, and then they are put away. What may or may not be explained the time is "why is a substance radioactive, what does it mean?"
Let's go straight back to the beginning of the periodic table, looking at the element Hydrogen. Hydrogen itself is quite unique in that it is the only element in existence which can possess an isotope containing only protons in the nucleus (Protium). The next isotope Deuterium contains one proton and one neutron and the 3rd isotope Tritium contains one proton and 2 neutrons. All other elements from helium onwards have nuclei which contain a varying number of protons and neutrons. We said previously that the number of protons sets the identity of the element, and the number of neutrons tells us the isotope. But what more can we learn from this?
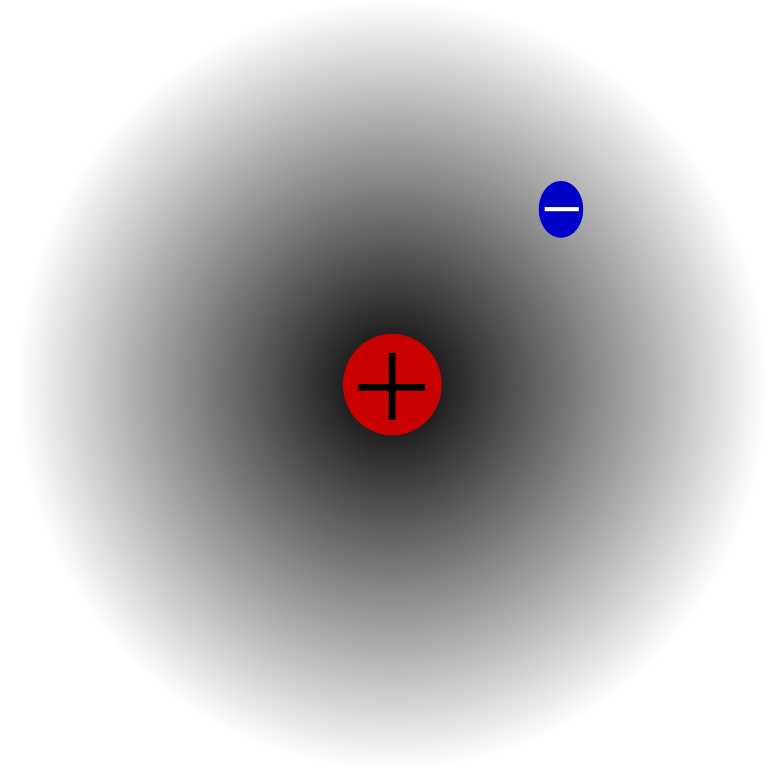
This particular depiction of a simple hydrogen atom shows the single electron in its "electron cloud" on its journey around the single proton nucleus. For reasons which I don't intend to explain at this level, this image is probably a more realistic model than the "electrons orbiting around the nucleus" model which you've become accustomed to.
As the nuclei get larger, more and more protons are added as are more and more neutrons. The whole atom is kept electrically neutral by the fact that the number of electrons in different energy levels around the nucleus always matches the number of protons in the nucleus. When the number of electrons varies, we get "ions" but generally the atom is neutral in its "unexcited" or "ground" state.
If you put two "north" poles, or two "south" of a magnet side-by-side, there will be a tendency for them to push away from each other as "likes repel".
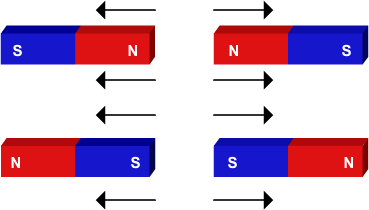
There is no reason why this shouldn't be the case when you start to pack more and more positive charges into nucleus.
How come therefore that in say, a Gold atom which contains 79 protons, the protons aren't pushing away from each other causing the nucleus to expand and fall apart?. We've already discovered the reason why the negatively charged electrons don't simply crash into the nucleus ("History of the Atom" - the Niels Bohr model) but we now need to consider why the nucleus doesn't simply fly apart? At GCSE level you don't really need to know much about this, but there are forces at work inside the nucleus called "Strong Nuclear Forces" which help to bind nucleus together, to overcome the urge for the protons to push away from each other.
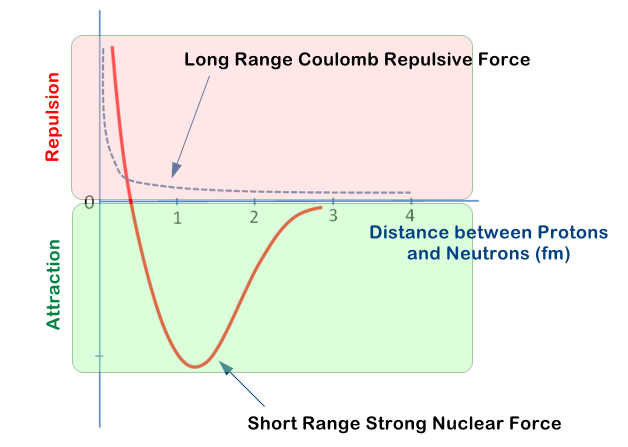
At very close distances, the strong nuclear force is repulsive (the pink shaded area), the protons (or nucleons) are kept at an optimum distance away from each other but as the distance between them grows the strong nuclear force reaches a maximum and then starts to die away (the green shaded area).
The strong nuclear force cannot hold together large nuclei, because it cannot overcome the much stronger Coulomb Repulsive Force and so as more and more neutrons and protons come together to form an elements nucleus, we face a situation where the nucleus starts to get too big to be held together by the strong nuclear force. These nuclei then become "unstable" and breakup, releasing nuclear particles and energy to return to a more stable state (usually a smaller nucleus).
In the following sections will take a look at the types of particles that can be "ejected" from a decaying nucleus.
>> Questions <<
Go To >> Radioactive Decay Pathways <<
Go To >> Appendix P2 - Radioactive Particle Penetration <<