Appendix P1 - Radioactive Decay Pathways
|
|
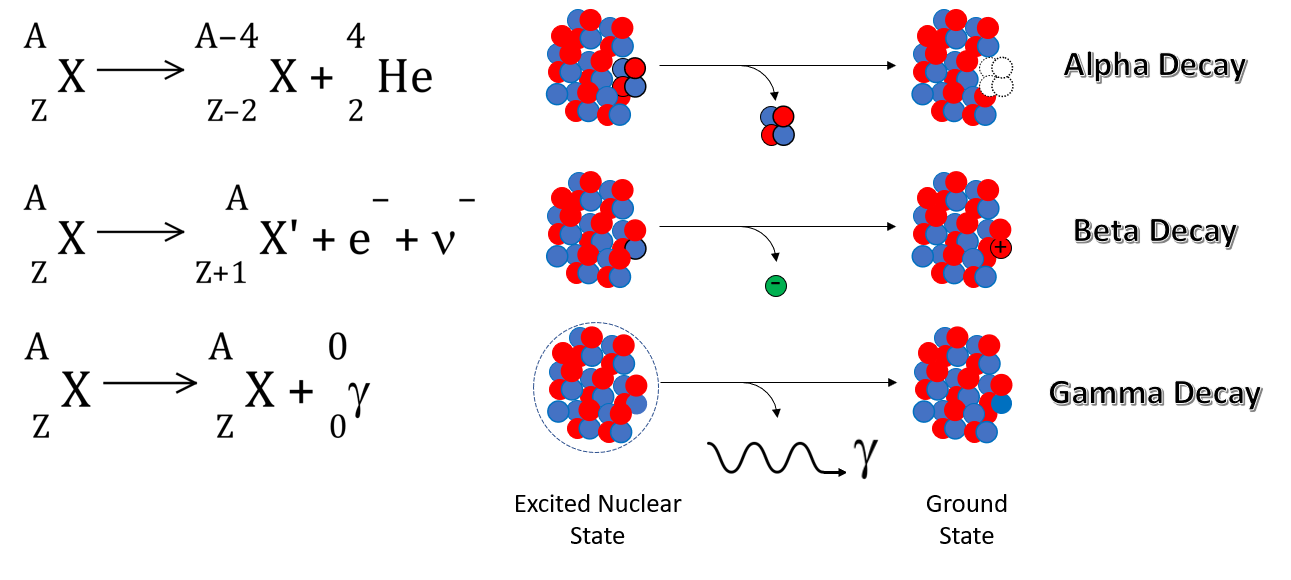
When the nucleus breaks up by alpha emission, 2 protons and 2 neutrons are ejected in the form of a doubly positive helium nucleus, or alpha particle. This causes the nucleus (atom) to decrease in atomic mass by 4 and in atomic number by 2. The nucleus then becomes an isotope of the element 2 spaces back in the periodic table. So for example when Uranium emits an alpha particle, it becomes an isotope of Thorium. |
|
|
When the nucleus undergoes beta emission, a neutron decays to a proton with the release of the corresponding negative charge in the form of an electron. The nucleus (atom) experiences no change in atomic mass but increases by one unit in atomic number, therefore the nucleus (atom) becomes an isotope of the next element up in the periodic table. |
|
|
When the nucleus undergoes gamma emission, there is no change in atomic mass or atomic number. The nucleus (atom) existing in an excited state, energetically, releases the excess energy in the form of a gamma ray, returning to the ground state itself. |
Back To >> Radioactivity <<
Go To >> Appendix P2 - Radioactive Particle Penetration <<


