Group 2 - The Alkaline Earth Metals
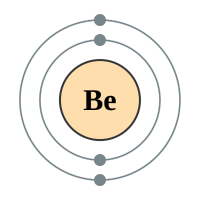
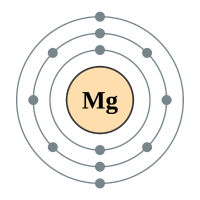
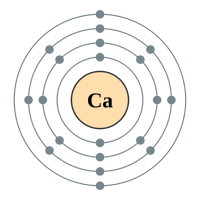
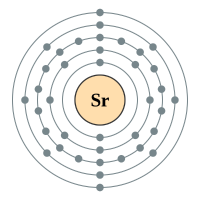
The alkaline earth metals form group two of the periodic table. We have seen previously that the way in which we can tell which group an element belongs depends on the number of electrons in the outermost shell of the atom. The group 2 elements all have two electrons in their outer shell.
|
|
|
|
|
Beryllium |
Magnesium |
Calcium ** |
|
2 shells - 4 electrons - [2,2] |
3 shells - 12 electrons - [2,8,2] |
4 shells - 20 electrons - [2,8,8,2) |
|
|
|
|
|
Strontium |
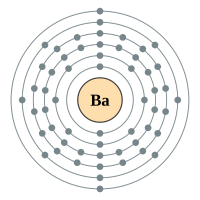
Barium |
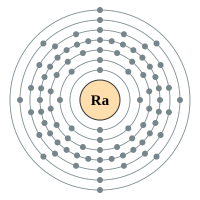
Radium |
|
5 shells - 38 electrons - [2,8,18,8,2] |
6 shells - 56 electrons - [2,8,18,18,8,2] |
7 shells - 88 electrons - [2,8,18,32,18,8,2] |
** Calcium is probably as high as you will go at GCSE, certainly when it comes to electron configurations.
Back To >> [A] Oxidation State <<