[A] Group 3-12 - Transition Elements
This section is mainly A Level stuff, but if you are really interested you might derive some benefit from it. It will explain why transition elements often have highly coloured ions in solution.
Lying between Group 2, the Alkaline Earth Metals and Group 3 (Boron, Aluminium Gallium Indium Thallium and Nihonium) sits a complete block of elements with certain unusual properties. Depending on the periodic table that you are looking at, these may just be referred to as the "D Block Elements" or groups 3 to 12 inclusive, with the aforementioned Group 3 (Boron and so forth) renamed to Group 13.
These are the Transition Elements, in older texts you may find them referred to as the Transition Metals, as they appear to be typical metals with similar properties. They are good conductors of heat and electricity, and very dense, strong and shiny. They are however, less reactive than the metals in Group 1 and 2.
Most of the transition "metals" conform ions with different oxidation states, and they can produce highly coloured complexes in solution. The reasons why these ions produce highly coloured complexes is beyond the intentions of the chemistry section of this document, but I will try to explain some of the main features in a fairly simplified way.
We need to briefly look at why these are called the D block elements before attempting to simplify the reasons for their highly coloured and multiple oxidation state behaviours. If we consider the element Calcium, you may be able to recall the electronic configuration as [2,8,8,2] which, in this notation explains the number of electrons and the fact that there are two in the outermost shell putting it in group 2. When you study chemistry to a higher level, you will expand on the "shells" method of electronic configuration to include "sub shells" which are labelled "s" "p" "d" and "f".
Let me just take another look at the electronic configuration of Calcium, but this time will give you in the "sub shell" format.
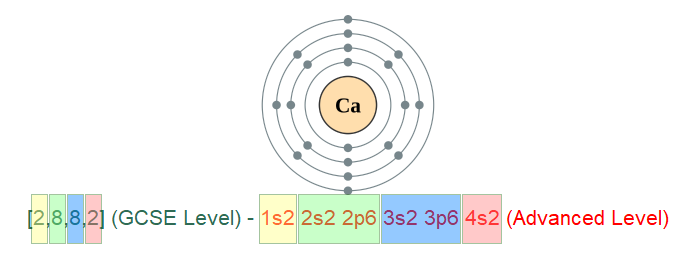
At GCSE level you can see four coloured boxes, holding the respective maximum permitted numbers of electrons (with the exception of shell number four, shown at GCSE level "pink 2"). The fourth shell, as you will see fairly soon, can in fact hold a maximum of 18 electrons. I mentioned before the "d" sub shells and of course the "f" sub shells, but there simply are not enough electrons to start to fill up the "d" shell until we get to the next element in the table, Scandium.
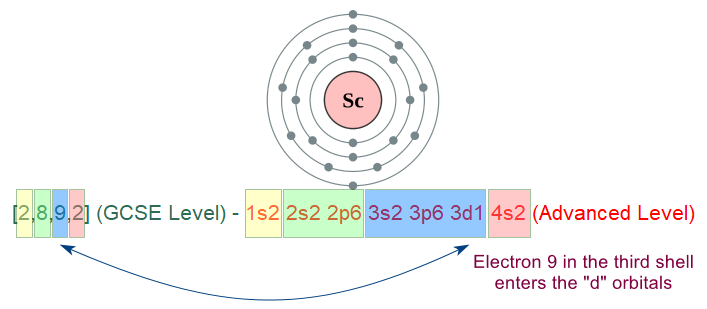
[2,8,9,2] (GCSE Level) - 1s2 2s2 2p6 3s2 3p6 3d1 4s2 (Advanced Level)
Now, for reasons that I don't want to go into too deeply, Scandium is not technically regarded as a transition element, neither is Zinc, but I'll briefly explain this in a short while.
Looking at the new "sub shell" concept that I introduced, in A-level chemistry we start to move away from the "solar system" thinking of electrons orbiting around the nucleus like the planets orbit around the sun. The red and blue areas in the D orbitals below are mathematical calculations identifying places in space relative to the nucleus of the atom, where a "d" electron is likely to be found (it's to do with probabilities).
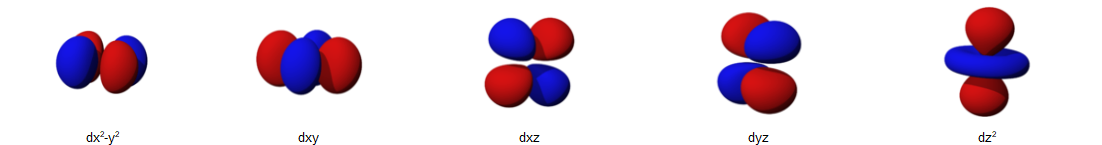
What we can say is that these five "probability areas" hopeful that the same energy level (they're all in the main shell number 3), but just to confuse things even more, this is going to change in a bit :-)
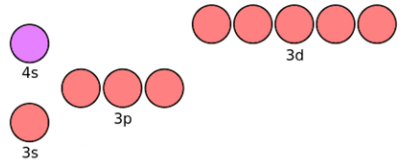
The above diagram is a section of the main "orbital filling hierarchy" that I placed in "Appendix C7 - Orbital SPDF Filling Hierarchy" and if you study it you will see that the 3s orbital has a lower energy than the 3p orbitals, the 3p orbitals have lower energy than the 4s orbital and the 3-D orbitals have the highest energy of all, despite the fact that they are in the third shell not the fourth shell like the 4s orbital. This therefore sets the order in which the shells will fill up with electrons, remember that each "orbital/circle" can only hold a maximum of two electrons, so for example if your 3p orbitals were full with six electrons, the next one would have to go into the 4s orbital.
All I need you to understand from this part, is that the 3d orbitals have all got the same energy.
Now imagine a transition element (metal) ion, let's select Cobalt. Here is the electronic configuration of Cobalt shown in the style of the diagram above:
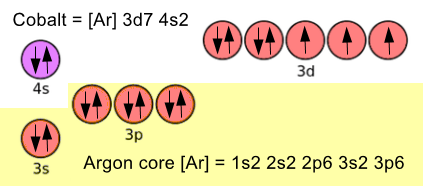
Don't worry too much the up and down arrows at this stage, there are rules which govern how the sub shells are filled, but these are way beyond the scope of GCSE.
Taking Cobalt as our "Transition Element of choice", there are two main oxidation states, that is Cobalt can exist as a 2+ or 3+ cation. In the first case (2+) Cobalt loses both of its "4s" electrons and in the second case (3+) and additional electron from the "3d" subshell.
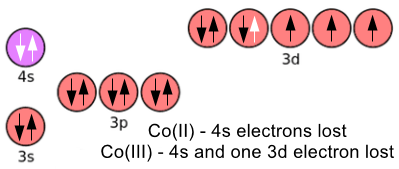
We now have Co2+ as [Ar] 3d7 and Co3+ as [Ar] 3d6.
Note here that the "paired" electron will be lost before any of the unpaired electrons (reasons are Advanced level) and we still have 6 or 7 "d" subshell electrons out of a possible 10. The ions are stable in their 2+ and 3+ oxidation states with a partially completed "d" subshell, this brings me to the accepted definition of a transition element:
A transition element (metal) is one that forms
stable ions that have incompletely filled d orbitals.
The transition elements are full of surprises, for example given the definition above, Scandium and Zinc in the first row of the transition elements, although in the "d" block are not technically transition elements because when they are in their usual ionic / oxidation state, in the case of Scandium the "d" subshell is completely empty, and in the case of Zinc the "d" subshell is full. The ions are stable but in these two cases the "d" subshell is not partly occupied (its either empty or full !).
So how do the transition elements make such beautiful coloured compounds?
Transition metals make "complexes", by "dative" bonding between negatively charged nonmetallic molecules such as Water, Ammonia et cetera. As you will recall in a "dative" bond, the bonding electrons are all provided by one party, in this case the negatively charged nonmetallic molecule, which is often referred to as a "ligand".
The "ligand field theory" or "crystal field theory" explains why transition metal complexes are coloured. First of all let's take a look at the "d" subshell, we will use Cobalt again:
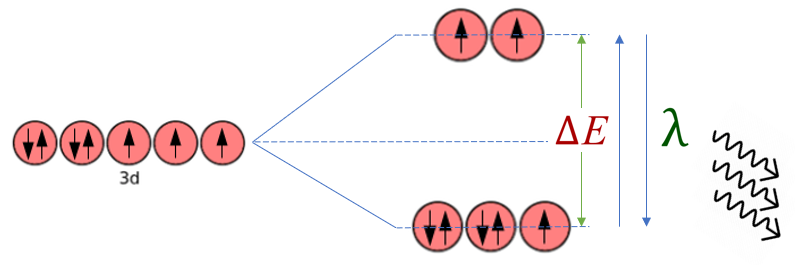
The left-hand side of the diagram shows Cobalt (II) as the "2+" ion, all of the "d" orbitals have the same energy level. When the complex is formed, due to the dative bonding between the metal cation and the "donated" electrons on the ligand, this causes a disruption to the "energetic status quo" of the "d" orbitals, reducing some energetically to a lower level, and raising others energetically to a higher level. This causes an energy (shown in the diagram) where an electron, through the absorption of energy, can be promoted to a higher energy level. When the electron decays back to its lower energy level, the energy absorbed is released as a photon of light. The energy level difference, and the type of ligand involved are contributory factors to the energy levels and therefore the energy of the photon released. This determines the colour of the light that is not absorbed by the complex, in other words the colour that you see.

Just to finish off this little detour into some quite complicated chemistry, here are some more transition metal complex colours:
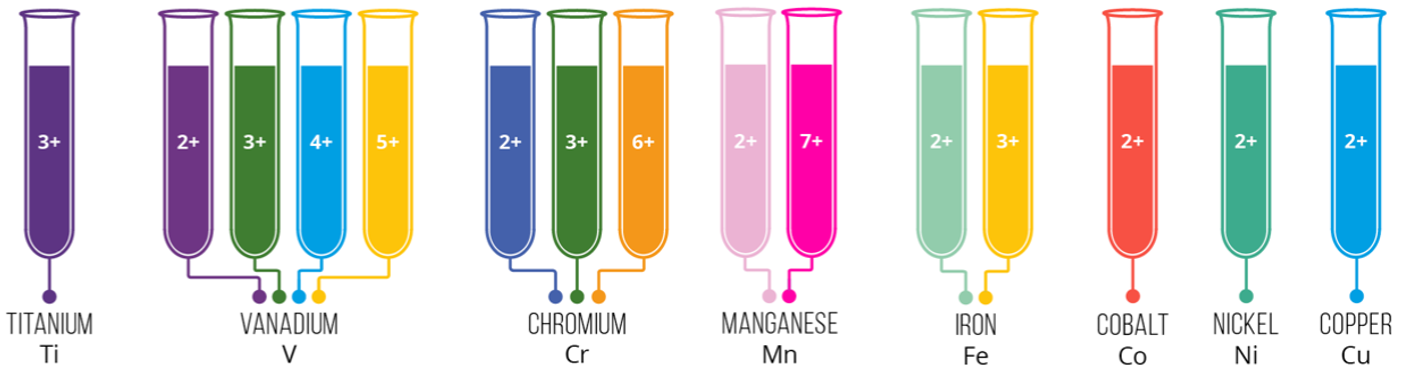
On the extreme right of the diagram, is the Cu(II) ion that I spoke about above, and the Co(II) ion, which is pink in aqueous solution (please understand that these colours are illustrative only and may vary slightly from what you would actually see if you were looking at a solution in a flask or beaker).
Now take a look at the ions produced by Chromium, particularly in the "3+" and "6+" ion.
Many years ago, in the 1970s and 1980s, in the United Kingdom and probably other countries, the reduction of the Cr (VI) to Cr (III) was of particular importance to law enforcement, as it was the underlying chemistry to the old "glass tube and bag" breathalyser.
If you drink intoxicants containing what we refer to as "alcohol", such as beer wine and spirits, you are in fact consuming "Ethanol" (old name Ethyl Alcohol). Alcohols can be oxidised with acidified solutions of Potassium Dichromate, which contains orange "Cr6+" ions. The oxidation of the alcohol is accompanied by a reduction (electron gain, remember OIL RIG) of this ion to "Cr3+":
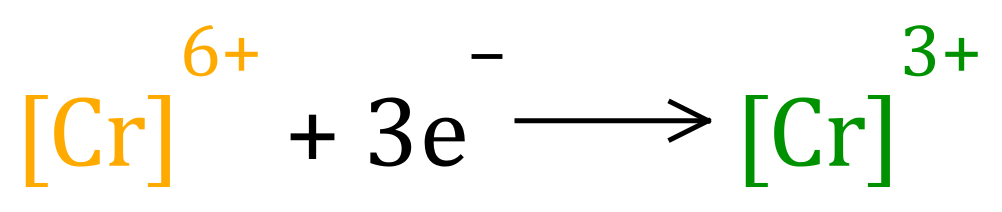
The amount of "alcohol" in your breath would determine how much green Cr3+ would be formed. A small ring etched on the side of the glass tube would indicate when you were likely to be "over the limit" and the polythene bag on the end of the tube make sure you produced a sufficiently large sample of breath. This method has of course been superseded by electronic versions now, but this is an interesting story showing the use of quite complicated chemical processes.
The full reaction is this: