Group 5/15 - The Pnictogens
A Pnictogen /ˈnɪktədʒən/ (from Ancient Greek: πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. This group is also known as the Nitrogen family. It consists of the elements Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), Bismuth (Bi), and perhaps the chemically uncharacterised synthetic element Moscovium (Mc).
|
|
|
|
|
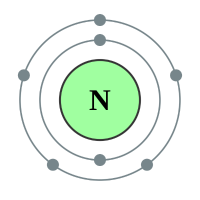
Nitrogen |
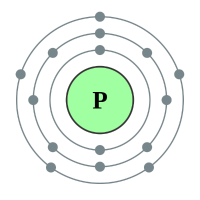
Phosphorous |
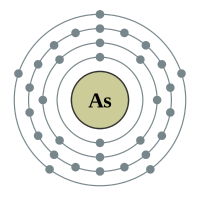
Arsenic |
|
2 shells - 5 electrons - [2,3] |
3 shells - 15 electrons - [2,8,5] |
4 shells - 33 electrons - [2,8,18,5) |
|
|
|
|
|
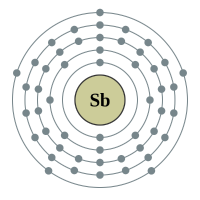
Antimony |
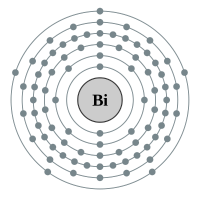
Bismuth |
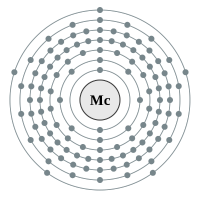
Moscovium |
|
5 shells - 51 electrons - [2,8,18,18,5] |
6 shells - 83 electrons - [2,8,18,32,18,5] |
7 shells - 115 electrons - [2,8,18,32,32,18,5] |
In modern IUPAC notation, it is called Group 15. In CAS and the old IUPAC systems it was called Group VA and Group VB respectively (pronounced "group five A" and "group five B", "V" for the Roman numeral 5). In the field of semiconductor physics, it is still usually called Group V.[3] The "five" ("V") in the historical names comes from the "pentavalency" of nitrogen, reflected by the stoichiometry of compounds such as N2O5.
They have also been called the Pentels.
Back To >> [A] Oxidation State <<