Group 0/8/18 - The Noble (Inert) Gases
The Group 8 (or Group 0, Group 18) elements are not very reactive, in fact initially they were regarded as the inert gases but developments in inorganic chemistry have seen at least one of the elements (Xenon) capable of forming compounds with fluorine. However it is also known that under very extreme conditions, the element Krypton can be forced to react with Fluorine to make a di-fluoride, and oxygenated compounds of Xenon and Fluorine have also been made to exist.
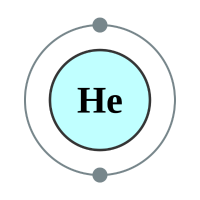
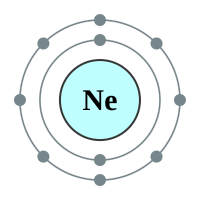
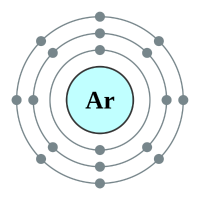
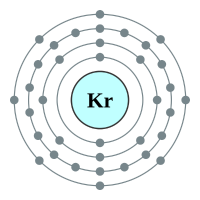
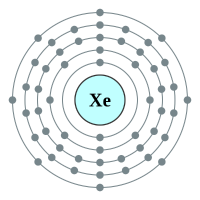
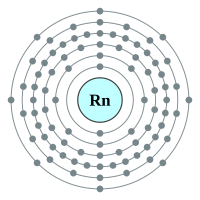
What makes the "inert" or as they regarding the "noble" gases so unreactive is the fact that they all possess a complete outer shell of electrons. With the exception of helium which can only hold two electrons in its outer (and only) shell, the remaining gases have a full "octet" and as such no desire to gain or lose further electrons. This gives them an energetically stable configuration. At room temperature and pressure all of the noble gases exist in a colourless mono atomic state.
|
|
|
|
|
|
|
|
|
1 shell - 2 electrons - [2] |
2 shells - 10 electrons - [2,8] |
3 shells - 18 electrons - [2,8,8] |
|
|
|
|
|
|
|
|
|
4 shells - 36 electrons - [2,8,18,8] |
5 shells - 54 electrons - [2,8,18,18,8] |
6 shells - 86 electrons - [2,8,18,32,18,8] |
|
|
|
|
|
|
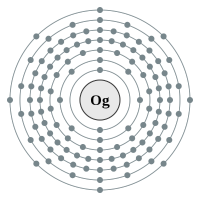
7 shells - 118 electrons - [2,8,18,32,32,18,8] |
|
|
|
|
|
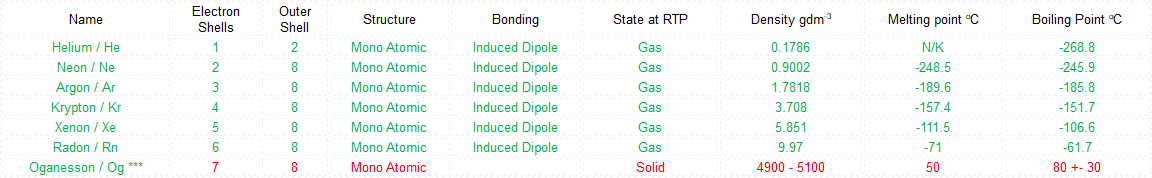
*** Predicted data in red.
As we proceed down the group, the boiling points of the noble gases increase. This is because the number of electrons is increasing and as such the inter-atomic forces between noble gas atoms will get stronger. More energy is required to disrupt these forces and so the boiling points increase.
>> Questions <<