Conservation of Mass
Q1.Calculate the mass of copper oxide formed when 127 g of copper reacts with 32 g of oxygen to form copper oxide
The first thing that you need to do in a situation like this is to establish the balanced chemical formula for the reaction.
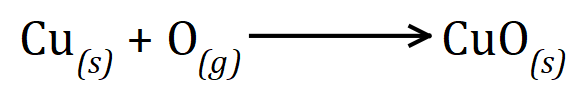
We can see that the reaction is one-to-one, that is one mole of copper will react with one mole of oxygen atoms (remember that this is half a mole of oxygen molecules) to give one mole of copper oxide. We are told that we have 127 g of copper which is 2 moles and 32 g of oxygen atoms which is also 2 moles. Logically then we should end up with 2 moles of copper oxide. The relative molecular mass of copper oxide is 63.5+16= 79.5 and so therefore if we double this we will end up with 159 g of copper oxide and this is the correct answer. Note that this answer could have simply been arrived at by adding the 2 masses together at the start but as the situation gets more and more complicated, it is probably best to get used to using molar quantities to start with.
Q2. During a chemical reaction, 56 g of nitrogen reacts with hydrogen to form 68 g of ammonia. What is the mass of hydrogen that reacts?
This question is becoming tricky already because we have an unknown quantity, here it will be useful to establish the balanced chemical reaction between nitrogen and hydrogen. In its simplest term:
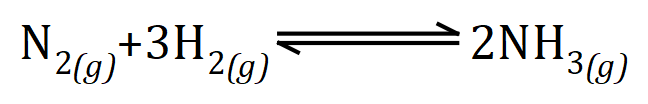
The reaction between nitrogen and hydrogen to produce ammonia is conducted during the Haber Process is reversible, but for the purposes of this mathematical problem we can overlook that technicality.
We can see that one mole of nitrogen molecules will react with 3 moles of hydrogen molecules to produce 2 moles of ammonia gas. We are told that we have 56 g of nitrogen gas which, in its diatomic form is 2 moles, and that we produce 68 g of ammonia. The relative molecular mass of ammonia is 17 therefore we produce 4 moles of ammonia. Stop at this point to reconsider what we have, 2 moles of nitrogen gas reacts with an unknown quantity of hydrogen gas to produce 4 moles of ammonia. from the equation we can see that 1 mole of nitrogen will react to 3 moles of hydrogen to produce 2 moles of ammonia, but we are told that we in fact have 2 moles of nitrogen which means that we must use 6 moles of hydrogen to produce the required 4 moles of ammonia which we're also told. 6 moles of hydrogen has a mass of 6 times (1×2) which equals 12 g. Therefore the answer to our question is that the mass of hydrogen reacting is 12 g. Again, from the law of conservation of mass we could have simply taken away 56 from 68 which would have given us a correct result anyway, but it is best as I said before to get used to using molar quantities and performing this type of arithmetic.
Q3. Chlorine reacts with sodium bromide according to the equation given at the beginning of the answer, use relative form of the masses of the reactants and products to show that mass is conserved during this reaction.
Examination questions wouldn't really be structured like this, but I've done it for ease of layout. The equation mentioned in the question is thus:
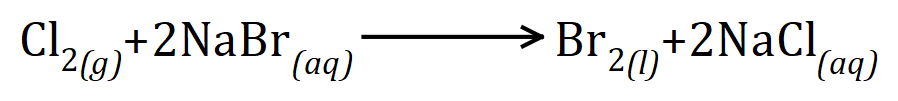
Incidentally this is a good example of a displacement reaction where chlorine, being the more reactive halogen of the 2 will displace bromine from its salt.
The relative molecular mass of chlorine is 71 and the relative molecular mass of sodium bromide is (23+80) the relative molecular mass of bromine in its diatomic form is 160 and that of NaCl's (23+35.5). Let us now substitute these numbers and the appropriate molar quantities into a simple mathematical equality to see if they do in fact balance out:
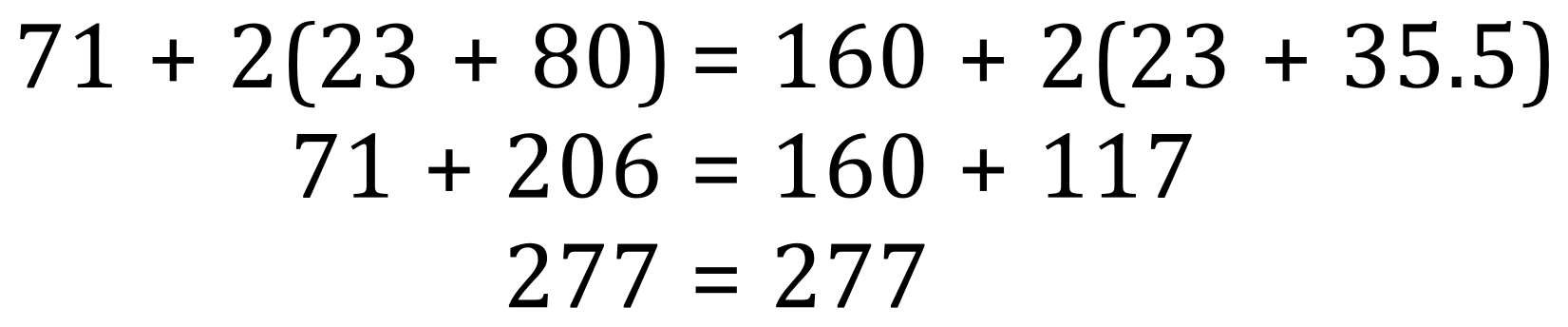
We can see from the simple arithmetic that as both sides show the same value, the law of conservation of mass is preserved.
Questions may not always require you to give a mathematical, finite response. You may be given a scenario and be asked to explain an observation. for example, consider the next question:
Q4. A student heated 5 g of calcium in an unsealed test tube so that it reacted with oxygen. At the end of the reaction the mass of the product inside the test tube was 7 g. Explain this observation.
In laboratory conditions, the oxygen that react with the calcium must have come from the atmosphere, this is made especially clear when you are told that the test tube is unsealed. The reaction would produce calcium oxide according to the following equation:
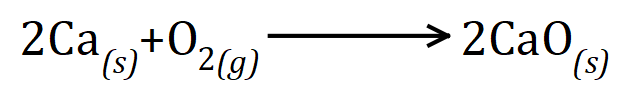
If you wish to stop at a simple observation you could do so by saying that the additional 2 g of mass has come from the oxygen in the atmosphere which is now combined with the calcium to produce the calcium oxide. If you wish to go a little bit further and show this mathematically you can do so by working out some molar quantities.
5 g of calcium, the relative atomic mass of which is 40, is 0.125 moles. We can see from the equation that 0.125 moles of calcium would require either 0.125 moles of oxygen atoms or 0.0625 moles of oxygen molecules. As long as you are consistent in your calculation it doesn't really matter which one you choose. I will stick to the moles/molecules (the latter) and assume that we use 0.0625 moles of oxygen molecules. the relative molecular mass of oxygen (diatomic) is 32, therefore one mole of oxygen molecules would have a mass of 32 g. We notice that our reactant increases in mass by 2 g as it turns into product, therefore this 2 g must be oxygen. How many moles of oxygen are contained in 2 g?
2÷32 = 0.0625 moles.
We have therefore taken our observation one step further forward and explained the laws of conservation of mass mathematically. It is likely that this would be an expectation in a higher GCSE chemistry paper.
Q5. Calcium carbonate decomposes when it is heated to form calcium oxide and carbon dioxide gas. The balanced equation for the reaction is as shown below:
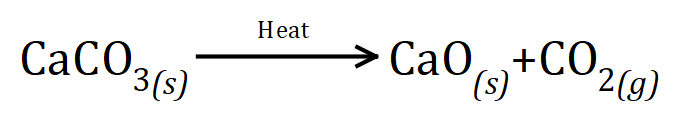
This is a multipart question............during an experiment, 14 g of calcium oxide and 11 g of carbon dioxide are formed:
(a) what mass of calcium carbonate was there at the beginning of the reaction
(b) predict the mass of calcium oxide that would be produced if the same reaction was carried out using 3 times as much calcium carbonate
(c) what volume would the carbon dioxide gas produced in this reaction occupy at RTP?
(d) calculate the relative formula masses of each of the substances in the reaction, the relative atomic masses C = 12, Ca = 40, O = 16.
(e) use your results to (d) above to show that the law of conservation of mass is upheld in this reaction.
(a) working simply on the law of conservation of mass, the mass of calcium carbonate at the beginning of the reaction must be the same as the total masses of the products, in this case 14+11 = 25 g
(b) we can see from the balanced chemical reaction that one mole of calcium carbonate produces one mole of calcium oxide and one mole of carbon dioxide during thermal decomposition, if we triple the amount of calcium carbonate then we must also triple the amount of calcium oxide that would be produced so in this case we would then produce 42 g of calcium oxide.
(c) we now know that one mole of any gas at RTP will occupy 24 dm3 . Furthermore we know that the relative molecular mass of carbon dioxide is 44, and that one mole would contain 44 g and as we have already said occupy 24 dm3 .The question tells us that we have in fact produced 11 g of carbon dioxide which is one quarter of a mole. As a result of this one quarter of a mole will take up obviously one quarter of the space that a full mole would take up. Therefore the volume of carbon dioxide produced during this reaction would be 6 dm3
(d) the relative formula mass of calcium carbonate is (40+12+(3 x 16)) = 100, the relative formula mass of calcium oxide is (40+16) = 56 and the relative formula mass of carbon dioxide we have already stated as 44, which is of course 12+16+16.
(e) we can see that the law of conservation of mass is upheld because on the left-hand side of the equation we have a substance with a relative formula mass of 100, and on the right-hand side we have 2 substances with an accumulative total mass of 56+44 = 100. As the amounts on either side of the equation are the same this proves that the law of conservation of mass is upheld.
Q6. More reactive halogens can displace less reactive halogens from solutions of their salts, for example chlorine is more reactive than bromine and would therefore be able to displace bromine from one of its salts, and similarly iodine would be displaced by bromine according to the reactivity going down group 7. If you are unsure about where this information comes from, study the halogens in your textbook and return to this question later. Otherwise if you feel confident just accept what you've been given and carry on with the calculation.
During a chemical reaction we wish to produce a mixture containing 12% bromine by mass.
This is a multipart question:
(a) what mass of calcium bromide would be needed to provide enough bromine to make 20 g of this mixture?
(b) the calcium bromide is made by reacting calcium iodide with bromine using the displacement mechanism shown in the equation below. Calculate the mass of calcium iodide that will react to form the mass of calcium bromide needed in the mixture.

First of all as we have the equation, let us look at the matter using molar quantities. The relative molecular mass of calcium iodide is 40+127+127= 294 and the molecular mass of bromine is 160 (80+80) the relative molecular mass of calcium bromide is 40+80+18=200 and the molecular mass of iodine is 254 (remember it is diatomic). Therefore we can state that 294 g of calcium iodide would require 160 g of bromine to produce 200 g of calcium bromide and 254 g of iodine. Does this satisfy the law of conservation of mass?, Well add up the numbers and make sure. Does 294+160= 200+254?, Well 294+160 is 454, and 200+254 is also 454 therefore the law of conservation of mass is upheld.
(a) 20 g of the mixture is required, the mixture will contain 12% bromine by mass so the amount of bromine in 20 g would be 20 x (12/100) = 2.4 g of bromine. The mass of bromine in calcium bromide by mass is 160 / 200 or 80%. We would therefore require 2.4÷0.8= 3 g of calcium bromide.
(b) we have already looked at the molar quantities, but now we know that we are going to be requiring 3 g of calcium bromide which is 3÷200= 0.015 moles. The equation is one-to-one in as much as one mole of calcium iodide will ultimately produce one mole of calcium bromide, therefore we can see that we would need to start with 0.015 moles of calcium iodide, which would have a mass of 0.015 x 294 = 4.4 g (to one decimal place).
Back To >> Questions <<
Back To >> Conservation Of Mass <<