Conservation of Mass
Q1.Calculate the mass of copper oxide formed when 127 g of copper reacts with 32 g of oxygen to form copper oxide
Q2. During a chemical reaction, 56 g of nitrogen reacts with hydrogen to form 68 g of ammonia. What is the mass of hydrogen that reacts?
Q3. Chlorine reacts with sodium bromide according to the equation given at the beginning of the answer, use relative form of the masses of the reactants and products to show that mass is conserved during this reaction.
Q4. A student heated 5 g of calcium in an unsealed test tube so that it reacted with oxygen. At the end of the reaction the mass of the product inside the test tube was 7 g. Explain this observation.
Q5. Calcium carbonate decomposes when it is heated to form calcium oxide and carbon dioxide gas. The balanced equation for the reaction is as shown below:
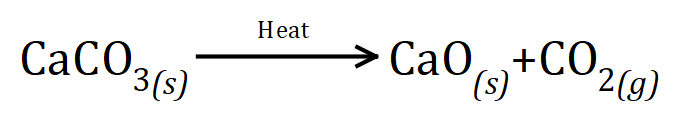
This is a multipart question............during an experiment, 14 g of calcium oxide and 11 g of carbon dioxide are formed:
(a) what mass of calcium carbonate was there at the beginning of the reaction
(b) predict the mass of calcium oxide that would be produced if the same reaction was carried out using 3 times as much calcium carbonate
(c) what volume would the carbon dioxide gas produced in this reaction occupy at RTP?
(d) calculate the relative formula masses of each of the substances in the reaction, the relative atomic masses C = 12, Ca = 40, O = 16.
(e) use your results to (d) above to show that the law of conservation of mass is upheld in this reaction.
Q6. More reactive halogens can displace less reactive halogens from solutions of their salts, for example chlorine is more reactive than bromine and would therefore be able to displace bromine from one of its salts, and similarly iodine would be displaced by bromine according to the reactivity going down group 7. If you are unsure about where this information comes from, study the halogens in your textbook and return to this question later. Otherwise if you feel confident just accept what you've been given and carry on with the calculation.
During a chemical reaction we wish to produce a mixture containing 12% bromine by mass.
This is a multipart question:
(a) what mass of calcium bromide would be needed to provide enough bromine to make 20 g of this mixture?
(b) the calcium bromide is made by reacting calcium iodide with bromine using the displacement mechanism shown in the equation below. Calculate the mass of calcium iodide that will react to form the mass of calcium bromide needed in the mixture.

Go To >> Solutions <<
Back To >> Conservation Of Mass <<